Age dependent accumulation patterns of advanced glycation end product receptor (RAGE) ligands and binding intensities between RAGE and its ligands differ in the liver, kidney, and skeletal muscle
- PMID: 28592983
- PMCID: PMC5460364
- DOI: 10.1186/s12979-017-0095-2
Age dependent accumulation patterns of advanced glycation end product receptor (RAGE) ligands and binding intensities between RAGE and its ligands differ in the liver, kidney, and skeletal muscle
Abstract
Background: Much evidence indicates receptor for advanced glycation end products (RAGE) related inflammation play essential roles during aging. However, the majority of studies have focused on advanced glycation end products (AGEs) and not on other RAGE ligands. In the present study, the authors evaluated whether the accumulation of RAGE ligands and binding intensities between RAGE and its ligands differ in kidney, liver, and skeletal muscle during aging.
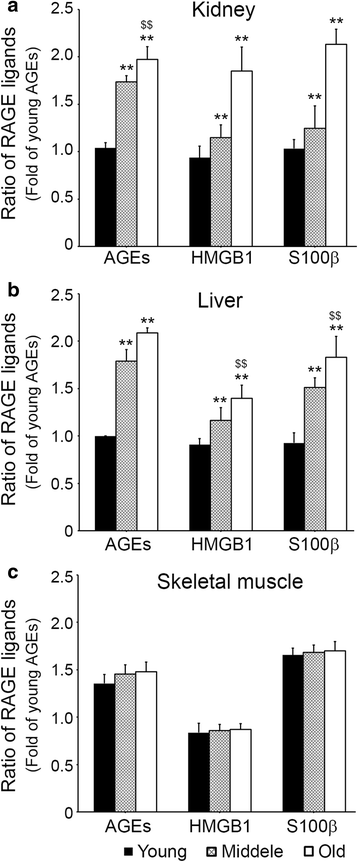
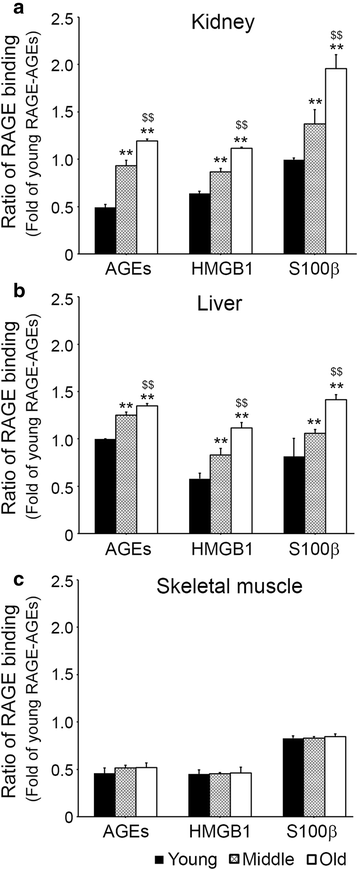
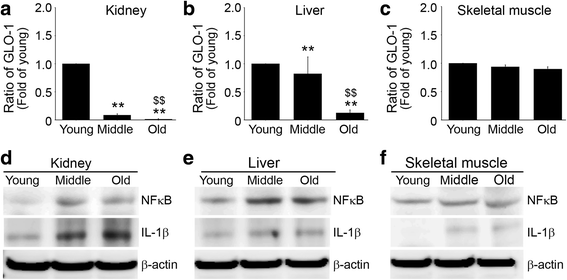
Results: In C57BL/6 N mice aged 12 weeks, 12 months, and 22 months, ligands accumulation, binding intensities between RAGE and its ligands, activated macrophage infiltration, M1/M2 macrophage expression, glyoxalase-1expression, and signal pathways related to inflammation were evaluated. The RAGE ligands age-associated accumulation patterns were found to be organ dependent. Binding intensities between RAGE and its ligands in kidney and liver increased with age, but those in skeletal muscle were unchanged. Infiltration of activated macrophages in kidney and liver increased with age, but infiltration in the skeletal muscle was unchanged. M1 expression increased and M2 and glyoxalase-1 expression decreased with age in kidney and liver, but their expressions in skeletal muscle were not changed.
Conclusion: These findings indicate patterns of RAGE ligands accumulation, RAGE/ligands binding intensities, or inflammation markers changes during aging are organs dependent.
Keywords: AGEs; Aging; Macrophage activation; RAGE; RAGE ligands.
Figures

References
-
- Schmidt AM, Vianna M, Gerlach M, et al. Isolation and characterization of two binding protein for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. - PubMed
-
- Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

