Streamlining recombination-mediated genetic engineering by validating three neutral integration sites in Synechococcus sp. PCC 7002
- PMID: 28592992
- PMCID: PMC5458483
- DOI: 10.1186/s13036-017-0061-8
Streamlining recombination-mediated genetic engineering by validating three neutral integration sites in Synechococcus sp. PCC 7002
Abstract
Background: Synechococcus sp. PCC 7002 (henceforth Synechococcus) is developing into a powerful synthetic biology chassis. In order to streamline the integration of genes into the Synechococcus chromosome, validation of neutral integration sites with optimization of the DNA transformation protocol parameters is necessary. Availability of BioBrick-compatible integration modules is desirable to further simplifying chromosomal integrations.
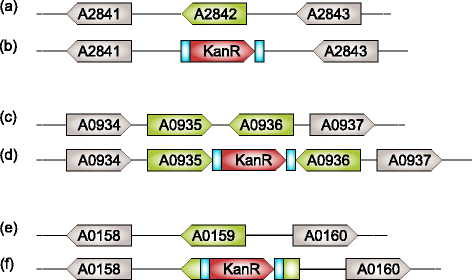
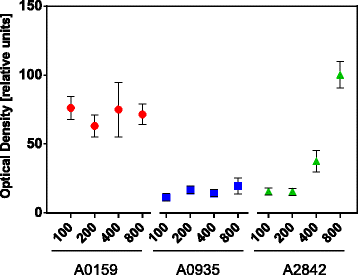
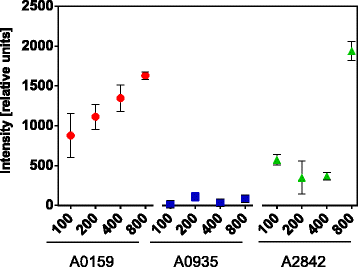
Results: We designed three BioBrick-compatible genetic modules, each targeting a separate neutral integration site, A2842, A0935, and A0159, with varying length of homologous region, spanning from 100 to 800 nt. The performance of the different modules for achieving DNA integration were tested. Our results demonstrate that 100 nt homologous regions are sufficient for inserting a 1 kb DNA fragment into the Synechococcus chromosome. By adapting a transformation protocol from a related cyanobacterium, we shortened the transformation procedure for Synechococcus significantly.
Conclusions: The optimized transformation protocol reported in this study provides an efficient way to perform genetic engineering in Synechococcus. We demonstrated that homologous regions of 100 nt are sufficient for inserting a 1 kb DNA fragment into the three tested neutral integration sites. Integration at A2842, A0935 and A0159 results in only a minimal fitness cost for the chassis. This study contributes to developing Synechococcus as the prominent chassis for future synthetic biology applications.
Keywords: BioBrick; Cyanobacteria; Genetic engineering; Neutral integration sites; Synechococcus sp. PCC7002; Synthetic biology; Transformation.
Figures

Similar articles
-
Genetic tools for advancement of Synechococcus sp. PCC 7002 as a cyanobacterial chassis.Microb Cell Fact. 2016 Nov 10;15(1):190. doi: 10.1186/s12934-016-0584-6. Microb Cell Fact. 2016. PMID: 27832791 Free PMC article.
-
Systematic identification of a neutral site on chromosome of Synechococcus sp. PCC7002, a promising photosynthetic chassis strain.J Biotechnol. 2019 Apr 10;295:37-40. doi: 10.1016/j.jbiotec.2019.02.007. Epub 2019 Mar 7. J Biotechnol. 2019. PMID: 30853638
-
Improving a Synechocystis-based photoautotrophic chassis through systematic genome mapping and validation of neutral sites.DNA Res. 2015 Dec;22(6):425-37. doi: 10.1093/dnares/dsv024. Epub 2015 Oct 21. DNA Res. 2015. PMID: 26490728 Free PMC article.
-
Expression of genes in cyanobacteria: adaptation of endogenous plasmids as platforms for high-level gene expression in Synechococcus sp. PCC 7002.Methods Mol Biol. 2011;684:273-93. doi: 10.1007/978-1-60761-925-3_21. Methods Mol Biol. 2011. PMID: 20960136
-
Towards the development of a new generation of whole-cell bioreporters to sense iron bioavailability in oceanic systems-learning from the case of Synechococcus sp. PCC7002 iron bioreporter.J Appl Microbiol. 2019 Nov;127(5):1291-1304. doi: 10.1111/jam.14277. Epub 2019 Jun 26. J Appl Microbiol. 2019. PMID: 30970168 Review.
Cited by
-
Development of a Biotechnology Platform for the Fast-Growing Cyanobacterium Synechococcus sp. PCC 11901.Biomolecules. 2022 Jun 23;12(7):872. doi: 10.3390/biom12070872. Biomolecules. 2022. PMID: 35883428 Free PMC article.
-
Insights into cyanobacterial alkane biosynthesis.J Ind Microbiol Biotechnol. 2022 Apr 14;49(2):kuab075. doi: 10.1093/jimb/kuab075. J Ind Microbiol Biotechnol. 2022. PMID: 34718648 Free PMC article. Review.
-
Genetic, Genomics, and Responses to Stresses in Cyanobacteria: Biotechnological Implications.Genes (Basel). 2021 Mar 29;12(4):500. doi: 10.3390/genes12040500. Genes (Basel). 2021. PMID: 33805386 Free PMC article. Review.
-
New Applications of Synthetic Biology Tools for Cyanobacterial Metabolic Engineering.Front Bioeng Biotechnol. 2019 Feb 27;7:33. doi: 10.3389/fbioe.2019.00033. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 30873404 Free PMC article. Review.
-
CyanoGate: A Modular Cloning Suite for Engineering Cyanobacteria Based on the Plant MoClo Syntax.Plant Physiol. 2019 May;180(1):39-55. doi: 10.1104/pp.18.01401. Epub 2019 Feb 28. Plant Physiol. 2019. PMID: 30819783 Free PMC article.
References
-
- Ungerer J, Tao L, Davis M, Ghirardi M, Maness P-C, Yu J. Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energy Environ Sci. 2012;5:8998. doi: 10.1039/c2ee22555g. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

