MicroRNA Regulation of Oxidative Stress-Induced Cellular Senescence
- PMID: 28593022
- PMCID: PMC5448073
- DOI: 10.1155/2017/2398696
MicroRNA Regulation of Oxidative Stress-Induced Cellular Senescence
Abstract
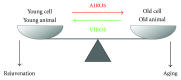
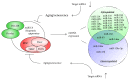
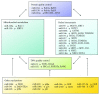
Aging is a time-related process of functional deterioration at cellular, tissue, organelle, and organismal level that ultimately brings life to end. Cellular senescence, a state of permanent cell growth arrest in response to cellular stress, is believed to be the driver of the aging process and age-related disorders. The free radical theory of aging, referred to as oxidative stress (OS) theory below, is one of the most studied aging promoting mechanisms. In addition, genetics and epigenetics also play large roles in accelerating and/or delaying the onset of aging and aging-related diseases. Among various epigenetic events, microRNAs (miRNAs) turned out to be important players in controlling OS, aging, and cellular senescence. miRNAs can generate rapid and reversible responses and, therefore, are ideal players for mediating an adaptive response against stress through their capacity to fine-tune gene expression. However, the importance of miRNAs in regulating OS in the context of aging and cellular senescence is largely unknown. The purpose of our article is to highlight recent advancements in the regulatory role of miRNAs in OS-induced cellular senescence.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

