Quantum chemical modeling of the reaction path of chorismate mutase based on the experimental substrate/product complex
- PMID: 28593134
- PMCID: PMC5458464
- DOI: 10.1002/2211-5463.12224
Quantum chemical modeling of the reaction path of chorismate mutase based on the experimental substrate/product complex
Abstract
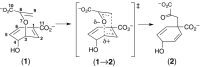
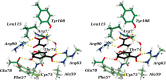
Chorismate mutase is a well-known model enzyme, catalyzing the Claisen rearrangement of chorismate to prephenate. Recent high-resolution crystal structures along the reaction coordinate of this enzyme enabled computational analyses at unprecedented detail. Using quantum chemical simulations, we investigated how the catalytic reaction mechanism is affected by electrostatic and hydrogen-bond interactions. Our calculations showed that the transition state (TS) was mainly stabilized electrostatically, with Arg90 playing the leading role. The effect was augmented by selective hydrogen-bond formation to the TS in the wild-type enzyme, facilitated by a small-scale local induced fit. We further identified a previously underappreciated water molecule, which separates the negative charges during the reaction. The analysis includes the wild-type enzyme and a non-natural enzyme variant, where the catalytic arginine was replaced with an isosteric citrulline residue.
Keywords: Claisen rearrangement; chorismate mutase; enzyme catalysis; pericyclic reaction; transition state stabilization.
Figures


References
-
- Sogo SG, Widlanski TS, Hoare JH, Grimshaw CE, Berchtold GA and Knowles JR (1984) Stereochemistry of the rearrangement of chorismate to prephenate: chorismate mutase involves a chair transition state. J Am Chem Soc 106, 2701–2703.
-
- Young IG, Gibson F and MacDonald CG (1969) Enzymic and nonenzymic transformations of chorismic acid and related cyclohexadienes. Biochim Biophys Acta 192, 62–72. - PubMed
-
- Gajewski JJ, Jurayj J, Kimbrough DR, Gande ME, Ganem B and Carpenter BK (1987) On the mechanism of rearrangement of chorismic acid and related compounds. J Am Chem Soc 109, 1170–1186.
-
- Copley SD and Knowles JR (1987) The conformational equilibrium of chorismate in solution: implications for the mechanism of the non‐enzymic and the enzyme‐catalyzed rearrangement of chorismate to prephenate. J Am Chem Soc 109, 5008–5013.
-
- Andrews PR, Smith GD and Young IG (1973) Transition‐state stabilization and enzymic catalysis. Kinetic and molecular orbital studies of the rearrangement of chorismate to prephenate. Biochemistry 12, 3492–3498. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

