Formulation and Development of a Validated UV-Spectrophotometric Analytical Method of Rutin Tablet
- PMID: 28593189
- PMCID: PMC5448154
- DOI: 10.1155/2017/2624947
Formulation and Development of a Validated UV-Spectrophotometric Analytical Method of Rutin Tablet
Abstract
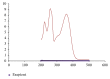
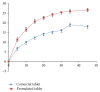
Rutin is available in some foods, fruits, and vegetables. It has various beneficial medical effects making it useful in the treatment of various diseases. Rutin is available in different oral dosage forms such as tablets or capsules, widely available in the market. Rutin and many herbal medicines lack quality control due to unavailability of analytical methods. In this study, we formulated rutin tablet and studied its stability using a simple developed analytical method. The dissolution profile of our formulated tablet was also inspected. The results showed that our developed method was linear (R2 = 0.999), precise (% RSD = 0.026), and accurate (% recovery = 98.55-103.34). The formulated rutin tablet was stable under accelerated conditions as well as room temperature for 150 days (% assay > 91.69). The dissolution profile over 45 minutes of our formulated tablet showed a better dissolution (26.5%) compared with the internationally marketed Rutin® tablet (18.5%). This study can serve as a guideline to companies that manufacture herbal products to improve their formulated herbs and apply validated analytical methods to check the quality of their product.
Figures


Similar articles
-
Formulation and development of Serratiopeptidase enteric coated tablets and analytical method validation by UV Spectroscopy.Int J Anal Chem. 2021 Oct 19;2021:9749474. doi: 10.1155/2021/9749474. eCollection 2021. Int J Anal Chem. 2021. PMID: 34712328 Free PMC article.
-
Tablet Formulation of a Synthesized Celecoxib Potassium Salt and Development of a Validated Method for Its Analysis.Curr Pharm Des. 2021;27(25):2872-2880. doi: 10.2174/1381612826666200904171940. Curr Pharm Des. 2021. PMID: 32887541
-
Synthesis of Rutin Derivatives to Enhance Lipid Solubility and Development of Topical Formulation with a Validated Analytical Method.Curr Drug Deliv. 2022;19(1):117-128. doi: 10.2174/1567201819666211220162535. Curr Drug Deliv. 2022. PMID: 34931961
-
Preparation and tableting of long-term stable amorphous rutin using porous silica.Eur J Pharm Biopharm. 2017 Apr;113:97-107. doi: 10.1016/j.ejpb.2016.11.009. Epub 2016 Nov 12. Eur J Pharm Biopharm. 2017. PMID: 27847275
-
Development of an oral rutin nanocrystal formulation.Int J Pharm. 2009 Mar 31;370(1-2):202-9. doi: 10.1016/j.ijpharm.2008.11.029. Epub 2008 Dec 7. Int J Pharm. 2009. PMID: 19114097
Cited by
-
Fabrication, optimization and characterization of an osmotic push-pull drug delivery system for paliperidone.J Taibah Univ Med Sci. 2023 Aug 30;18(6):1511-1518. doi: 10.1016/j.jtumed.2023.08.002. eCollection 2023 Dec. J Taibah Univ Med Sci. 2023. PMID: 37693824 Free PMC article.
-
Rech. f. & Esfand as adjunctive therapy for eradication: a randomized controlled trial.J Tradit Chin Med. 2025 Apr;45(2):437-442. doi: 10.19852/j.cnki.jtcm.2025.02.009. J Tradit Chin Med. 2025. PMID: 40151130 Free PMC article. Clinical Trial.
-
Enhanced Antioxidant, Anti-Aging, Anti-Tyrosinase, and Anti-Inflammatory Properties of Vanda coerulea Griff. Ex Lindl. Protocorm through Elicitations with Chitosan.Plants (Basel). 2024 Jun 26;13(13):1770. doi: 10.3390/plants13131770. Plants (Basel). 2024. PMID: 38999610 Free PMC article.
-
Formulation and development of Serratiopeptidase enteric coated tablets and analytical method validation by UV Spectroscopy.Int J Anal Chem. 2021 Oct 19;2021:9749474. doi: 10.1155/2021/9749474. eCollection 2021. Int J Anal Chem. 2021. PMID: 34712328 Free PMC article.
-
ULK1 Mediated Autophagy-Promoting Effects of Rutin-Loaded Chitosan Nanoparticles Contribute to the Activation of NF-κB Signaling Besides Inhibiting EMT in Hep3B Hepatoma Cells.Int J Nanomedicine. 2024 May 18;19:4465-4493. doi: 10.2147/IJN.S443117. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38779103 Free PMC article.
References
-
- Xu H., Li Y., Tang H.-W., Liu C.-M., Wu Q.-S. Determination of rutin with UV-Vis spectrophotometric and laser-induced fluorimetric detections using a non-scanning spectrometer. Analytical Letters. 2010;43(6):893–904. doi: 10.1080/00032710903488795. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
