Real-World Effectiveness of Antipsychotic Treatments in a Nationwide Cohort of 29 823 Patients With Schizophrenia
- PMID: 28593216
- PMCID: PMC5710250
- DOI: 10.1001/jamapsychiatry.2017.1322
Real-World Effectiveness of Antipsychotic Treatments in a Nationwide Cohort of 29 823 Patients With Schizophrenia
Abstract
Importance: It has remained unclear whether there are clinically meaningful differences between antipsychotic treatments with regard to preventing relapse of schizophrenia, owing to the impossibility of including large unselected patient populations in randomized clinical trials, as well as residual confounding from selection biases in observational studies.
Objective: To study the comparative real-world effectiveness of antipsychotic treatments for patients with schizophrenia.
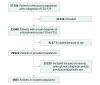
Design, setting, and participants: Prospectively gathered nationwide databases were linked to study the risk of rehospitalization and treatment failure from July 1, 2006, to December 31, 2013, among all patients in Sweden with a schizophrenia diagnosis who were 16 to 64 years of age in 2006 (29 823 patients in the total prevalent cohort; 4603 in the incident cohort of newly diagnosed patients). Within-individual analyses were used for primary analyses, in which each individual was used as his or her own control to eliminate selection bias. Traditional Cox proportional hazards multivariate regression was used for secondary analyses.
Main outcomes and measures: Risk of rehospitalization and treatment failure (defined as psychiatric rehospitalization, suicide attempt, discontinuation or switch to other medication, or death).
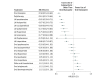
Results: There were 29 823 patients (12 822 women and 17 001 men; mean [SD] age, 44.9 [12.0] years). During follow-up, 13 042 of 29 823 patients (43.7%) were rehospitalized, and 20 225 of 28 189 patients (71.7%) experienced treatment failure. The risk of psychiatric rehospitalization was the lowest during monotherapy with once-monthly long-acting injectable paliperidone (hazard ratio [HR], 0.51; 95% CI, 0.41-0.64), long-acting injectable zuclopenthixol (HR, 0.53; 95% CI, 0.48-0.57), clozapine (HR, 0.53; 95% CI, 0.48-0.58), long-acting injectable perphenazine (HR, 0.58; 95% CI, 0.52-0.65), and long-acting injectable olanzapine (HR, 0.58; 95% CI, 0.44-0.77) compared with no use of antipsychotic medication. Oral flupentixol (HR, 0.92; 95% CI, 0.74-1.14), quetiapine (HR, 0.91; 95% CI, 0.83-1.00), and oral perphenazine (HR, 0.86; 95% CI, 0.77-0.97) were associated with the highest risk of rehospitalization. Long-acting injectable antipsychotic medications were associated with substantially lower risk of rehospitalization compared with equivalent oral formulations (HR, 0.78; 95% CI, 0.72-0.84 in the total cohort; HR, 0.68; 95% CI, 0.53-0.86 in the incident cohort). Clozapine (HR, 0.58; 95% CI, 0.53-0.63) and all long-acting injectable antipsychotic medications (HRs 0.65-0.80) were associated with the lowest rates of treatment failure compared with the most widely used medication, oral olanzapine. The results of several sensitivity analyses were consistent with those of the primary analyses.
Conclusions and relevance: Clozapine and long-acting injectable antipsychotic medications were the pharmacologic treatments with the highest rates of prevention of relapse in schizophrenia. The risk of rehospitalization is about 20% to 30% lower during long-acting injectable treatments compared with equivalent oral formulations.
Conflict of interest statement
Figures

Comment in
-
Clozapine and long-acting injectable antipsychotics reduce hospitalisation and treatment failure risk in patients with schizophrenia.Evid Based Ment Health. 2018 Aug;21(3):e11. doi: 10.1136/ebmental-2018-300003. Epub 2018 Jul 11. Evid Based Ment Health. 2018. PMID: 29997153 Free PMC article. No abstract available.
References
-
- Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60(6):553-564. - PubMed
-
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41. - PubMed
-
- Leucht S, Cipriani A, Spineli L, et al. . Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. - PubMed
-
- Hofer A, Hummer M, Huber R, Kurz M, Walch T, Fleischhacker WW. Selection bias in clinical trials with antipsychotics. J Clin Psychopharmacol. 2000;20(6):699-702. - PubMed
-
- Baandrup L, Gasse C, Jensen VD, et al. . Antipsychotic polypharmacy and risk of death from natural causes in patients with schizophrenia: a population-based nested case-control study. J Clin Psychiatry. 2010;71(2):103-108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

