Impact of hemodynamic goal-directed resuscitation on mortality in adult critically ill patients: a systematic review and meta-analysis
- PMID: 28593456
- PMCID: PMC5943381
- DOI: 10.1007/s10877-017-0032-0
Impact of hemodynamic goal-directed resuscitation on mortality in adult critically ill patients: a systematic review and meta-analysis
Abstract
The effect of hemodynamic optimization in critically ill patients has been challenged in recent years. The aim of the meta-analysis was to evaluate if a protocolized intervention based on the result of hemodynamic monitoring reduces mortality in critically ill patients. We performed a systematic review and meta-analysis according to the Cochrane Handbook for Systematic Reviews of Interventions. The study was registered in the PROSPERO database (CRD42015019539). Randomized controlled trials published in English, reporting studies on adult patients treated in an intensive care unit, emergency department or equivalent level of care were included. Interventions had to be protocolized and based on results from hemodynamic measurements, defined as cardiac output, stroke volume, stroke volume variation, oxygen delivery, and central venous-or mixed venous oxygenation. The control group had to be treated without any structured intervention based on the parameters mentioned above, however, monitoring by central venous pressure measurements was allowed. Out of 998 screened papers, thirteen met the inclusion criteria. A total of 3323 patients were enrolled in the six trials with low risk of bias (ROB). The mortality was 22.4% (374/1671 patients) in the intervention group and 22.9% (378/1652 patients) in the control group, OR 0.94 with a 95% CI of 0.73-1.22. We found no statistically significant reduction in mortality from hemodynamic optimization using hemodynamic monitoring in combination with a structured algorithm. The number of high quality trials evaluating the effect of protocolized hemodynamic management directed towards a meaningful treatment goal in critically ill patients in comparison to standard of care treatment is too low to prove or exclude a reduction in mortality.
Keywords: Critical care; Fluid therapy; Hemodynamic monitoring; Meta-analysis; Mortality; Protocol.
Conflict of interest statement
Conflict of interest
On behalf of all authors, the corresponding author states that there are no competing interests.
Research involving human participants
No ethical permission was sought as the study did not involve human participants.
Figures
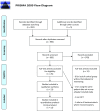
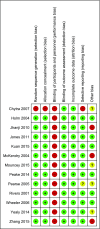
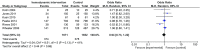

References
-
- Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, Sander M, Spies C, Lefrant JY, De Backer D. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41:1529–1537. doi: 10.1007/s00134-015-3850-x. - DOI - PMC - PubMed
-
- Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, Sander M, Spies C, Lefrant JY, De Backer D, Investigators F, Group ET Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intens Care Med. 2015;41:1529–1537. doi: 10.1007/s00134-015-3850-x. - DOI - PMC - PubMed
-
- Boulain T, Boisrame-Helms J, Ehrmann S, Lascarrou JB, Bougle A, Chiche A, Lakhal K, Gaudry S, Perbet S, Desachy A, Cabasson S, Geneau I, Courouble P, Clavieras N, Massanet PL, Bellec F, Falquet Y, Reminiac F, Vignon P, Dequin PF, Meziani F. Volume expansion in the first 4 days of shock: a prospective multicentre study in 19 French intensive care units. Intensive Care Med. 2015;41:248–256. doi: 10.1007/s00134-014-3576-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

