Nutrient acquisition strategies of mammalian cells
- PMID: 28593971
- PMCID: PMC5541675
- DOI: 10.1038/nature22379
Nutrient acquisition strategies of mammalian cells
Abstract
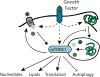
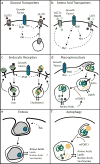
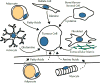
Mammalian cells are surrounded by diverse nutrients, such as glucose, amino acids, various macromolecules and micronutrients, which they can import through transmembrane transporters and endolysosomal pathways. By using different nutrient sources, cells gain metabolic flexibility to survive periods of starvation. Quiescent cells take up sufficient nutrients to sustain homeostasis. However, proliferating cells depend on growth-factor-induced increases in nutrient uptake to support biomass formation. Here, we review cellular nutrient acquisition strategies and their regulation by growth factors and cell-intrinsic nutrient sensors. We also discuss how oncogenes and tumour suppressors promote nutrient uptake and thereby support the survival and growth of cancer cells.
Figures

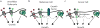
References
-
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. W.H. Freeman 6th edition. 2012
-
- Eagle H. Nutrition needs of mammalian cells in tissue culture. Science (New York, N.Y.) 1955;122:501–514. - PubMed
-
- Alberts BM, et al. Molecular biology of the cell. Garland Science; 6th edition. 2014
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

