Insights into Hunter syndrome from the structure of iduronate-2-sulfatase
- PMID: 28593992
- PMCID: PMC5472762
- DOI: 10.1038/ncomms15786
Insights into Hunter syndrome from the structure of iduronate-2-sulfatase
Abstract
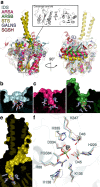
Hunter syndrome is a rare but devastating childhood disease caused by mutations in the IDS gene encoding iduronate-2-sulfatase, a crucial enzyme in the lysosomal degradation pathway of dermatan sulfate and heparan sulfate. These complex glycosaminoglycans have important roles in cell adhesion, growth, proliferation and repair, and their degradation and recycling in the lysosome is essential for cellular maintenance. A variety of disease-causing mutations have been identified throughout the IDS gene. However, understanding the molecular basis of the disease has been impaired by the lack of structural data. Here, we present the crystal structure of human IDS with a covalently bound sulfate ion in the active site. This structure provides essential insight into multiple mechanisms by which pathogenic mutations interfere with enzyme function, and a compelling explanation for severe Hunter syndrome phenotypes. Understanding the structural consequences of disease-associated mutations will facilitate the identification of patients that may benefit from specific tailored therapies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Lin H. Y. et al.. Incidence of the mucopolysaccharidoses in Taiwan, 1984–2004. Am. J. Med. Genet. A 149A, 960–964 (2009). - PubMed
-
- Baehner F. et al.. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J. Inherit. Metab. Dis. 28, 1011–1017 (2005). - PubMed
-
- Froissart R. et al.. Identification of iduronate sulfatase gene alterations in 70 unrelated Hunter patients. Clin. Genet. 53, 362–368 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

