Glucose targets for preventing diabetic kidney disease and its progression
- PMID: 28594069
- PMCID: PMC6481869
- DOI: 10.1002/14651858.CD010137.pub2
Glucose targets for preventing diabetic kidney disease and its progression
Abstract
Background: Diabetes is the leading cause of end-stage kidney disease (ESKD) around the world. Blood pressure lowering and glucose control are used to reduce diabetes-associated disability including kidney failure. However there is a lack of an overall evidence summary of the optimal target range for blood glucose control to prevent kidney failure.
Objectives: To evaluate the benefits and harms of intensive (HbA1c < 7% or fasting glucose levels < 120 mg/dL versus standard glycaemic control (HbA1c ≥ 7% or fasting glucose levels ≥ 120 mg/dL for preventing the onset and progression of kidney disease among adults with diabetes.
Search methods: We searched the Cochrane Kidney and Transplant Specialised Register up to 31 March 2017 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: Randomised controlled trials evaluating glucose-lowering interventions in which people (aged 14 year or older) with type 1 or 2 diabetes with and without kidney disease were randomly allocated to tight glucose control or less stringent blood glucose targets.
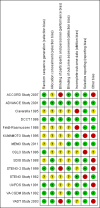
Data collection and analysis: Two authors independently assessed studies for eligibility and risks of bias, extracted data and checked the processes for accuracy. Outcomes were mortality, cardiovascular complications, doubling of serum creatinine (SCr), ESKD and proteinuria. Confidence in the evidence was assessing using GRADE. Summary estimates of effect were obtained using a random-effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) and 95% CI for continuous outcomes.
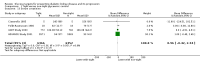
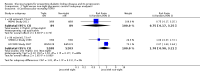
Main results: Fourteen studies involving 29,319 people with diabetes were included and 11 studies involving 29,141 people were included in our meta-analyses. Treatment duration was 56.7 months on average (range 6 months to 10 years). Studies included people with a range of kidney function. Incomplete reporting of key methodological details resulted in uncertain risks of bias in many studies. Using GRADE assessment, we had moderate confidence in the effects of glucose lowering strategies on ESKD, all-cause mortality, myocardial infarction, and progressive protein leakage by kidney disease and low or very low confidence in effects of treatment on death related to cardiovascular complications and doubling of serum creatinine (SCr).For the primary outcomes, tight glycaemic control may make little or no difference to doubling of SCr compared with standard control (4 studies, 26,874 participants: RR 0.84, 95% CI 0.64 to 1.11; I2= 73%, low certainty evidence), development of ESKD (4 studies, 23,332 participants: RR 0.62, 95% CI 0.34 to 1.12; I2= 52%; low certainty evidence), all-cause mortality (9 studies, 29,094 participants: RR 0.99, 95% CI 0.86 to 1.13; I2= 50%; moderate certainty evidence), cardiovascular mortality (6 studies, 23,673 participants: RR 1.19, 95% CI 0.73 to 1.92; I2= 85%; low certainty evidence), or sudden death (4 studies, 5913 participants: RR 0.82, 95% CI 0.26 to 2.57; I2= 85%; very low certainty evidence). People who received treatment to achieve tighter glycaemic control probably experienced lower risks of non-fatal myocardial infarction (5 studies, 25,596 participants: RR 0.82, 95% CI 0.67 to 0.99; I2= 46%, moderate certainty evidence), onset of microalbuminuria (4 studies, 19,846 participants: RR 0.82, 95% CI 0.71 to 0.93; I2= 61%, moderate certainty evidence), and progression of microalbuminuria (5 studies, 13,266 participants: RR 0.59, 95% CI 0.38 to 0.93; I2= 75%, moderate certainty evidence). In absolute terms, tight versus standard glucose control treatment in 1,000 adults would lead to between zero and two people avoiding non-fatal myocardial infarction, while seven adults would avoid experiencing new-onset albuminuria and two would avoid worsening albuminuria.
Authors' conclusions: This review suggests that people who receive intensive glycaemic control for treatment of diabetes had comparable risks of kidney failure, death and major cardiovascular events as people who received less stringent blood glucose control, while experiencing small clinical benefits on the onset and progression of microalbuminuria and myocardial infarction. The adverse effects of glycaemic management are uncertain. Based on absolute treatment effects, the clinical impact of targeting an HbA1c < 7% or blood glucose < 6.6 mmol/L is unclear and the potential harms of this treatment approach are largely unmeasured.
Conflict of interest statement
None known.
Figures



























Comment in
-
Review: In diabetes, intensive and standard glycemic control do not differ for end-stage kidney disease or death.Ann Intern Med. 2017 Oct 17;167(8):JC47. doi: 10.7326/ACPJC-2017-167-8-047. Ann Intern Med. 2017. PMID: 29049766 No abstract available.
-
Intensive glucose control in patients with diabetes prevents onset and progression of microalbuminuria, but effects on end-stage kidney disease are still uncertain.Evid Based Med. 2017 Dec;22(6):219-220. doi: 10.1136/ebmed-2017-110806. Epub 2017 Nov 2. Evid Based Med. 2017. PMID: 29097446 No abstract available.
Similar articles
-
Glucagon-like peptide 1 (GLP-1) receptor agonists for people with chronic kidney disease and diabetes.Cochrane Database Syst Rev. 2025 Feb 18;2(2):CD015849. doi: 10.1002/14651858.CD015849.pub2. Cochrane Database Syst Rev. 2025. PMID: 39963952
-
Insulin and glucose-lowering agents for treating people with diabetes and chronic kidney disease.Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011798. doi: 10.1002/14651858.CD011798.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246878 Free PMC article.
-
Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients.Cochrane Database Syst Rev. 2017 Feb 27;2(2):CD009966. doi: 10.1002/14651858.CD009966.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jul 30;8:CD009966. doi: 10.1002/14651858.CD009966.pub3. PMID: 28238223 Free PMC article. Updated.
-
Immunosuppressive treatment for primary membranous nephropathy in adults with nephrotic syndrome.Cochrane Database Syst Rev. 2021 Nov 15;11(11):CD004293. doi: 10.1002/14651858.CD004293.pub4. Cochrane Database Syst Rev. 2021. PMID: 34778952 Free PMC article.
-
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease.Cochrane Database Syst Rev. 2023 Jul 19;7(7):CD007751. doi: 10.1002/14651858.CD007751.pub3. Cochrane Database Syst Rev. 2023. PMID: 37466151 Free PMC article.
Cited by
-
Association between dietary fiber intake and diabetic nephropathy among adult diabetes mellitus in the United States: A cross-sectional study.Heliyon. 2024 Apr 23;10(9):e30036. doi: 10.1016/j.heliyon.2024.e30036. eCollection 2024 May 15. Heliyon. 2024. PMID: 38707305 Free PMC article.
-
Cardiovascular and renal outcomes by baseline albuminuria status and renal function: Results from the LEADER randomized trial.Diabetes Obes Metab. 2020 Nov;22(11):2077-2088. doi: 10.1111/dom.14126. Epub 2020 Aug 7. Diabetes Obes Metab. 2020. PMID: 32618386 Free PMC article. Clinical Trial.
-
Effects of Linagliptin on Cardiovascular and Kidney Outcomes in People With Normal and Reduced Kidney Function: Secondary Analysis of the CARMELINA Randomized Trial.Diabetes Care. 2020 Aug;43(8):1803-1812. doi: 10.2337/dc20-0279. Epub 2020 May 22. Diabetes Care. 2020. PMID: 32444457 Free PMC article. Clinical Trial.
-
Low-grade albuminuria in adult and elderly individuals with diabetes mellitus and arterial hypertension accompanied by Primary Health Care.Sci Rep. 2021 Sep 2;11(1):17565. doi: 10.1038/s41598-021-96652-6. Sci Rep. 2021. PMID: 34475440 Free PMC article.
-
Asian Pacific Society of Nephrology Clinical Practice Guideline on Diabetic Kidney Disease-2025 Update.Nephrology (Carlton). 2025 Jul;30 Suppl 2(Suppl 2):3-56. doi: 10.1111/nep.70030. Nephrology (Carlton). 2025. PMID: 40631380 Free PMC article. No abstract available.
References
References to studies included in this review
ACCORD Study 2007 {published data only}
-
- ACCORD Study Group, ACCORD Eye Study Group, Chew EY, Ambrosius WT, Davis MD, Danis RP, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes.[Erratum appears in N Engl J Med. 2011 Jan 13;364(2):190], [Erratum appears in N Engl J Med. 2012 Dec 20;367(25):2458]. New England Journal of Medicine 2010;363(3):233‐44. [MEDLINE: ] - PMC - PubMed
-
- ACCORD Study Group, Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. American Journal of Cardiology 2007;99(12A):21i‐33i. [MEDLINE: ] - PubMed
ADVANCE Study 2001 {published and unpublished data}
-
- Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high‐risk individuals with type 2 diabetes mellitus. Action in Diabetes and Vascular Disease: Preterax and diamicron modified‐release controlled evaluation. Journal of Hypertension ‐ Supplement 2001;19(4):S21‐8. [MEDLINE: ] - PubMed
-
- ADVANCE Collaborative Group. ADVANCE‐‐Action in Diabetes and Vascular Disease: patient recruitment and characteristics of the study population at baseline. Diabetic Medicine 2005;22(7):882‐8. [MEDLINE: ] - PubMed
-
- ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2008;358(24):2560‐72. [MEDLINE: ] - PubMed
-
- ADVANCE Management Committee. Study rationale and design of ADVANCE: action in diabetes and vascular disease‐‐Preterax and Diamicron MR Controlled Evaluation. Diabetologia 2001;44(9):1118‐20. [MEDLINE: ] - PubMed
-
- Blomster JI, Woodward M, Zoungas S, Hillis GS, Harrap S, Neal B, et al. The harms of smoking and benefits of smoking cessation in women compared with men with type 2 diabetes: an observational analysis of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron modified release Controlled Evaluation) trial. BMJ Open 2016;6(1):e009668. [MEDLINE: ] - PMC - PubMed
Ciavarella 1985 {published data only}
-
- Ciavarella A, Vannini P, Flammini M, Bacci L, Forlani G, Borgnino LC. Effect of long‐term near‐normoglycemia on the progression of diabetic nephropathy. Diabete et Metabolisme 1985;11(1):3‐8. [MEDLINE: ] - PubMed
DCCT 1986 {published data only}
-
- Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes 1986;35(5):530‐45. [MEDLINE: ] - PubMed
-
- Diabetes Control and Complications Trial (DCCT). Update. DCCT Research Group. Diabetes Care 1990;13(4):427‐33. [MEDLINE: ] - PubMed
-
- Diabetes Control and Complications Trial (DCCT): results of feasibility study. The DCCT Research Group. Diabetes Care 1987;10(1):1‐19. [MEDLINE: ] - PubMed
-
- Effect of intensive diabetes treatment on the development and progression of long‐term complications in adolescents with insulin‐dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Journal of Pediatrics 1994;125(2):177‐88. [MEDLINE: ] - PubMed
-
- Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney International 1995;47(6):1703‐20. [MEDLINE: ] - PubMed
Feldt‐Rasmussen 1986 {published data only}
-
- Feldt‐Rasmussen B, Mathiesen ER, Deckert T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin‐dependent diabetes. Lancet 1986;2(8519):1300‐4. [MEDLINE: ] - PubMed
-
- Feldt‐Rasmussen B, Mathiesen ER, Hegedus L, Deckert T. Kidney function during 12 months of strict metabolic control in insulin‐dependent diabetic patients with incipient nephropathy. New England Journal of Medicine 1986;314(11):665‐70. [MEDLINE: ] - PubMed
KUMAMOTO Study 1995 {published data only}
-
- Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Research & Clinical Practice 1995;28(2):103‐17. [MEDLINE: ] - PubMed
-
- Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long‐term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000;23 Suppl 2:B21‐9. [MEDLINE: ] - PubMed
-
- Wake N, Hisashige A, Katayama T, Kishikawa H, Ohkubo Y, Sakai M, et al. Cost‐effectiveness of intensive insulin therapy for type 2 diabetes: a 10‐year follow‐up of the Kumamoto study. Diabetes Research & Clinical Practice 2000;48(3):201‐10. [MEDLINE: ] - PubMed
MEMO Study 2011 {published data only}
-
- Crasto W, Jarvis J, Khunti K, Skinner TC, Gray LJ, Brela J, et al. Multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: the Microalbuminuria Education and Medication Optimisation (MEMO) study. Diabetes Research & Clinical Practice 2011;93(3):328‐36. [MEDLINE: ] - PubMed
-
- Crasto W, Khunti K, Jarvis KJ, Skinner TC, Gray LJ, Brela J, et al. Impact of intensive multi‐factorial intervention on novel markers of inflammation and vascular stiffness [abstract no: P446]. Diabetic Medicine 2011;28(Suppl 1):166. [EMBASE: 70631246]
-
- Crasto WA, Jarvis J, Brela J, Daly H, Gray LJ, Troughton J, et al. Interim analysis of the effects of a multifactorial intervention in people with type 2 diabetes and microalbuminuria after twelve months [abstract no: P410]. Diabetic Medicine 2009;26(Suppl 1):160. [EMBASE: 70342536]
OSLO Study 1986 {published data only}
-
- Dahl‐Jorgensen K, Brinchmann‐Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, et al. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. British Medical Journal Clinical Research Ed 1986;293(6556):1195‐9. [MEDLINE: ] - PMC - PubMed
-
- Dahl‐Jorgensen K, Hanssen KF, Kierulf P, Bjoro T, Sandvik L, Aagenaes O. Reduction of urinary albumin excretion after 4 years of continuous subcutaneous insulin infusion in insulin‐dependent diabetes mellitus. The Oslo Study. Acta Endocrinologica 1988;117(1):19‐25. [MEDLINE: ] - PubMed
SDIS Study 1988 {published data only}
-
- Jensen‐Urstad K, Reichard P, Jensen‐Urstad M. Decreased heart rate variability in patients with type 1 diabetes mellitus is related to arterial wall stiffness. Journal of Internal Medicine 1999;245(1):57‐61. [MEDLINE: ] - PubMed
-
- Johansson J, Reichard P, Jensen‐Urstad K, Rosfors S, Jensen‐Urstad M. Influence of glucose control, lipoproteins, and haemostasis function on brachial endothelial reactivity and carotid intima‐media area, stiffness and diameter in Type 1 diabetes mellitus patients. European Journal of Clinical Investigation 2003;33(6):472‐9. [MEDLINE: ] - PubMed
-
- Rathsman B, Jensen‐Urstad K, Nystrom T. Intensified insulin treatment is associated with improvement in skin microcirculation and ischaemic foot ulcer in patients with type 1 diabetes mellitus: a long‐term follow‐up study. Diabetologia 2014;57(8):1703‐10. [MEDLINE: ] - PubMed
-
- Reichard P. Risk factors for progression of microvascular complications in the Stockholm Diabetes Intervention Study (SDIS). Diabetes Research & Clinical Practice 1992;16(2):151‐6. [MEDLINE: ] - PubMed
-
- Reichard P, Berglund B, Britz A, Cars I, Nilsson BY, Rosenqvist U. Intensified conventional insulin treatment retards the microvascular complications of insulin‐dependent diabetes mellitus (IDDM): the Stockholm Diabetes Intervention Study (SDIS) after 5 years. Journal of Internal Medicine 1991;230(2):101‐8. [MEDLINE: ] - PubMed
STENO‐2 Study 1999 {published data only}
-
- Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. New England Journal of Medicine 2008;358(6):580‐91. [MEDLINE: ] - PubMed
-
- Gaede P, Parving H, Pedersen O. Multifactorial intervention in patients with type 2 diabetes: long‐term effects on mortality and vascular complications [abstract no: SA‐FC042]. Journal of the American Society of Nephrology 2007;18(Abstracts):43A. [CENTRAL: CN‐00740461]
-
- Gaede P, Vedel P, Larsen N, Jensen G, Parving HH, Pedersen O. The STENO‐2 study: intensified multifactorial intervention reduces the risk of cardiovascular disease in patients with type 2 diabetes and microalbuminuria [abstract no: F‐FC031]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):72a. [CENTRAL: CN‐00445410]
-
- Gaede P, Vedel P, Larsen N, Jensen G, Parving HH, Pedersen O. The Steno‐2 study: intensified multifactorial intervention reduces the risk of cardiovascular disease in patients with type 2 diabetes and microalbuminuria [abstract no: 181]. 38th Annual Meeting of the European Association for the Study of Diabetes (EASD); 2002 Sept 1‐5; Budapest, Hungary. 2002.
STENO Study 1982 {published data only}
-
- Effect of 6 months of strict metabolic control on eye and kidney function in insulin‐dependent diabetics with background retinopathy. Steno Study Group. Lancet 1982;1(8264):121‐4. [MEDLINE: ] - PubMed
UKPDS Study 1991 {published data only}
-
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group.[Erratum appears in Lancet 1999 Aug;354(9178):602]. Lancet 1998;352(9131):837‐53. [MEDLINE: ] - PubMed
-
- UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia 1991;34(12):877‐90. [MEDLINE: ] - PubMed
-
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9‐year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non‐insulin‐dependent diabetes mellitus. Annals of Internal Medicine 1996;124(1 pt 2):136‐45. [MEDLINE: ] - PubMed
VA‐CSDM Study 1992 {published data only}
-
- Abraira C, Colwell J, Nuttall F, Sawin CT, Henderson W, Comstock JP, et al. Cardiovascular events and correlates in the Veterans Affairs Diabetes Feasibility Trial. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes. Archives of Internal Medicine 1997;157(2):181‐8. [MEDLINE: ] - PubMed
-
- Abraira C, Colwell JA, Nuttall FQ, Sawin CT, Nagel NJ, Comstock JP, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care 1995;18(8):1113‐23. [MEDLINE: ] - PubMed
-
- Abraira C, Emanuele N, Colwell J, Henderson W, Comstock J, Levin S, et al. Glycemic control and complications in type II diabetes. Design of a feasibility trial. VA CS Group (CSDM). Diabetes Care 1992;15(11):1560‐71. [MEDLINE: ] - PubMed
-
- Abraira C, Henderson WG, Colwell JA, Nuttall FQ, Comstock JP, Emanuele NV, et al. Response to intensive therapy steps and to glipizide dose in combination with insulin in type 2 diabetes. VA feasibility study on glycemic control and complications (VA CSDM). Diabetes Care 1998;21(4):574‐9. [MEDLINE: ] - PubMed
-
- Abraira C, McGuire DK. Intensive insulin therapy in patients with type 2 diabetes: implications of the Veterans affairs (VA CSDM) feasibility trial. American Heart Journal 1999;138(5 Pt 1):S360‐5. [MEDLINE: ] - PubMed
VADT Study 2003 {published data only}
-
- Abraira C, Duckworth W, McCarren M, Emanuele N, Arca D, Reda D, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. Journal of Diabetes & its Complications 2003;17(6):314‐22. [MEDLINE: ] - PubMed
-
- Abraira C, Duckworth WC, Moritz T, VADT Group. Glycaemic separation and risk factor control in the Veterans Affairs Diabetes Trial: an interim report. Diabetes, Obesity & Metabolism 2009;11(2):150‐6. [MEDLINE: ] - PubMed
-
- Alele JD, Luttrell LM, Hollis BW, Luttrell DK, Hunt KJ, VADT Study Group. Relationship between vitamin D status and incidence of vascular events in the Veterans Affairs Diabetes Trial. Atherosclerosis 2013;228(2):502‐7. [MEDLINE: ] - PubMed
References to studies excluded from this review
Christiansen 1987 {published data only}
-
- Christiansen JS, Ingerslev J, Bernvil SS, Christensen CK, Hermansen K, Schmitz A. Near normoglycemia for 1 year has no effect on platelet reactivity, factor VIII, and von Willebrand factor in insulin‐dependent diabetes mellitus: a controlled trial. Journal of Diabetic Complications 1987;1(3):100‐6. [MEDLINE: ] - PubMed
Holman 1983 {published data only}
-
- Holman RR, Dornan TL, Mayon‐White V, Howard‐Williams J, Orde‐Peckar C, Jenkins L, et al. Prevention of deterioration of renal and sensory‐nerve function by more intensive management of insulin‐dependent diabetic patients. A two‐year randomised prospective study. Lancet 1983;1(8318):204‐8. [MEDLINE: ] - PubMed
Kawamori 1991 {published data only}
-
- Kawamori R, Kamado K, Kamada T. Recent progress in the treatment of diabetic nephropathy: importance of strict glycemic control on the regression of diabetic nephropathy. Journal of Diabetic Complications 1991;5(2‐3):88‐90. [MEDLINE: ] - PubMed
Wiseman 1985 {published data only}
-
- Wiseman MJ, Saunders AJ, Keen H, Viberti G. Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin‐dependent diabetes. New England Journal of Medicine 1985;312(10):617‐21. [MEDLINE: ] - PubMed
Additional references
CDC 2011
-
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf (accessed 8 April 2017).
Desouza 2003
-
- Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 2003;26(5):1485‐9. [MEDLINE: ] - PubMed
Duckworth 2009
-
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes.[Erratum appears in N Engl J Med. 2009 Sep 3;361(10):1028], [Erratum appears in N Engl J Med. 2009 Sep 3;361(10):1024‐5; PMID: 19726779]. New England Journal of Medicine 2009;360(2):129‐39. [MEDLINE: ] - PubMed
Finne 2005
-
- Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen‐Riska C. Incidence of end‐stage renal disease in patients with type 1 diabetes. JAMA 2005;294(14):1782‐7. [MEDLINE: ] - PubMed
Gerstain 2008
GRADE 2008
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] - PubMed
Hemmingsen 2011
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
IDF 2015
-
- International Diabetes Federation. IDF Diabetes atlas (7th edition), 2015. www.diabetesatlas.org/ (accessed 8 April 2017).
Ismail 2010
-
- Ismail‐Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial.[Erratum appears in Lancet. 2010 Oct 30;376(9751):1466]. Lancet 2010;376(9739):419‐30. [MEDLINE: ] - PMC - PubMed
Krolewski 1995
-
- Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin‐dependent diabetes mellitus. New England Journal of Medicine 1995;332(19):1251‐5. [MEDLINE: ] - PubMed
Moritz 2009
-
- Moritz T, Duckworth W, Abraira C. Veterans affairs diabetes trial ‐ corrections.[Erratum for N Engl J Med. 2009 Jan 8;360(2):129‐39; PMID: 19092145]. New England Journal of Medicine 2009;361(10):1024–5. [MEDLINE: ] - PubMed
Murray 2012
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years(DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010.[Erratum appears in Lancet. 2013 Feb 23;381(9867):628 Note: AlMazroa, Mohammad A [added]; Memish, Ziad A [added]]. Lancet 2012;380(9859):2197‐223. [MEDLINE: ] - PubMed
Patel 2008
-
- ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2008;358(24):2560‐72. [MEDLINE: ] - PubMed
Paterson 1998
-
- Paterson BL, Thorne S, Dewis M. Adapting to and managing diabetes. Image ‐ the Journal of Nursing Scholarship 1998;30(1):57‐62. [MEDLINE: ] - PubMed
Pavkov 2006
-
- Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth‐onset type 2 diabetes mellitus on incidence of end‐stage renal disease and mortality in young and middle‐aged Pima Indians. JAMA 2006;26(296):421‐6. [MEDLINE: ] - PubMed
Ritz 1999
-
- Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. New England Journal of Medicine 1999;341(15):1127‐33. [MEDLINE: ] - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
UKPDS 1998
-
- UK Prospective Diabetes Study Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group.[Erratum appears in Lancet 1999 Aug 14;354(9178):602]. Lancet 1998;352(9131):837‐53. [MEDLINE: ] - PubMed
USRDS 2011
-
- US Renal Data System. USRDS 2011 Annual Data Report: Atlas of End‐Stage Renal Disease in the United States. National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2011. www.usrds.org/atlas11.aspx (accessed 8 April 2017).
USRDS 2013
-
- US Renal Data System. 2013 atlas of CKD and ESRD. www.usrds.org/atlas13.aspx (accessed 8 April 2017).
Wild 2004
-
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047‐53. [MEDLINE: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

