An Effective, Versatile, and Inexpensive Device for Oxygen Uptake Measurement
- PMID: 28594349
- PMCID: PMC5483868
- DOI: 10.3390/jcm6060058
An Effective, Versatile, and Inexpensive Device for Oxygen Uptake Measurement
Abstract
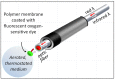
In the last ten years, the use of fluorescent probes developed to measure oxygen has resulted in several marketed devices, some unreasonably expensive and with little flexibility. We have explored the use of the effective, versatile, and inexpensive Redflash technology to determine oxygen uptake by a number of different biological samples using various layouts. This technology relies on the use of an optic fiber equipped at its tip with a membrane coated with a fluorescent dye (www.pyro-science.com). This oxygen-sensitive dye uses red light excitation and lifetime detection in the near infrared. So far, the use of this technology has mostly been used to determine oxygen concentration in open spaces for environmental studies, especially in aquatic media. The oxygen uptake determined by the device can be easily assessed in small volumes of respiration medium and combined with the measurement of additional parameters, such as lactate excretion by intact cells or the membrane potential of purified mitochondria. We conclude that the performance of by this technology should make it a first choice in the context of both fundamental studies and investigations for respiratory chain deficiencies in human samples.
Keywords: glycolysis; mitochondriopathy; oxygen uptake; respiration assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

