Compound heterozygous mutations in glycyl-tRNA synthetase (GARS) cause mitochondrial respiratory chain dysfunction
- PMID: 28594869
- PMCID: PMC5464557
- DOI: 10.1371/journal.pone.0178125
Compound heterozygous mutations in glycyl-tRNA synthetase (GARS) cause mitochondrial respiratory chain dysfunction
Abstract
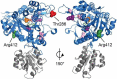
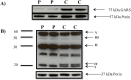
Glycyl-tRNA synthetase (GARS; OMIM 600287) is one of thirty-seven tRNA-synthetase genes that catalyses the synthesis of glycyl-tRNA, which is required to insert glycine into proteins within the cytosol and mitochondria. To date, eighteen mutations in GARS have been reported in patients with autosomal-dominant Charcot-Marie-Tooth disease type 2D (CMT2D; OMIM 601472), and/or distal spinal muscular atrophy type V (dSMA-V; OMIM 600794). In this study, we report a patient with clinical and biochemical features suggestive of a mitochondrial respiratory chain (MRC) disorder including mild left ventricular posterior wall hypertrophy, exercise intolerance, and lactic acidosis. Using whole exome sequencing we identified compound heterozygous novel variants, c.803C>T; p.(Thr268Ile) and c.1234C>T; p.(Arg412Cys), in GARS in the proband. Spectrophotometric evaluation of the MRC complexes showed reduced activity of Complex I, III and IV in patient skeletal muscle and reduced Complex I and IV activity in the patient liver, with Complex IV being the most severely affected in both tissues. Immunoblot analysis of GARS protein and subunits of the MRC enzyme complexes in patient fibroblast extracts showed significant reduction in GARS protein levels and Complex IV. Together these studies provide evidence that the identified compound heterozygous GARS variants may be the cause of the mitochondrial dysfunction in our patient.
Conflict of interest statement
Figures

References
-
- Kawakami N, Komatsu K, Yamashita H, Uemura K, Oka N, Takashima H, et al. (2014) [A novel mutation in glycyl-tRNA synthetase caused Charcot-Marie-Tooth disease type 2D with facial and respiratory muscle involvement]. Rinsho Shinkeigaku 54: 911–915. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

