Conophylline inhibits non-alcoholic steatohepatitis in mice
- PMID: 28594915
- PMCID: PMC5464552
- DOI: 10.1371/journal.pone.0178436
Conophylline inhibits non-alcoholic steatohepatitis in mice
Abstract
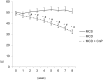
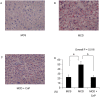
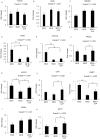
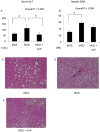
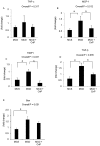
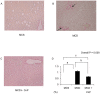
Conophylline (CnP), a vinca alkaloid extracted from the leaves of the tropical plant Ervatamia microphylla, attenuates hepatic fibrosis in mice. However, little is known about whether CnP inhibits steatosis, inflammation, and fibrosis in non-alcoholic steatohepatitis (NASH) in mice. A methionine-choline-deficient (MCD) diet was administered to male db/db mice as a NASH model, and CnP (1 μg/kg/d) was co-administered. Eight weeks after the commencement of the MCD diet, hepatic steatosis, inflammation, and fibrosis, and hepatic fat metabolism-, inflammation-, and fibrosis-related markers were examined. Feeding on an MCD for 8 weeks induced hepatic steatosis, inflammation, and fibrosis. CnP significantly attenuated the MCD-induced increases in hepatic steatosis, as well as hepatic inflammation and fibrosis. The MCD diet increased hepatic transforming growth factor-β (TGF-β) mRNA levels, which are correlated with hepatic steatosis, inflammation, and fibrosis. The diet also attenuated acyl-coenzyme A oxidase 1 (ACOX1) and carnitine palmitoyltransferase 1 (CPT1) mRNA levels, which are involved in β-oxidation. The putative mechanism of the CnP effect involves reduced hepatic TGF-β mRNA levels, and increased mRNA levels of hepatic peroxisome proliferator-activated receptor (PPAR) α and its target genes ACOX1 and CPT1. The results of this study indicate that CnP inhibits steatohepatitis, possibly through the inhibition of hepatic TGF-β mRNA levels, and induces an increase in PPARα mRNA levels, resulting in the attenuation of hepatic steatosis, inflammation, and fibrosis in mice. CnP might accordingly be a suitable therapeutic option for NASH.
Conflict of interest statement
Figures

References
-
- Almeda-Valdes P, Cuevas-Ramos D, Aguilar-Salinas CA. Metabolic syndrome and non-alcoholic fatty liver disease. Ann Hepatol. 2009;8 Suppl 1:S18–24. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

