The introduction of mesenchymal stromal cells induces different immunological responses in the lungs of healthy and M. tuberculosis infected mice
- PMID: 28594940
- PMCID: PMC5464766
- DOI: 10.1371/journal.pone.0178983
The introduction of mesenchymal stromal cells induces different immunological responses in the lungs of healthy and M. tuberculosis infected mice
Abstract
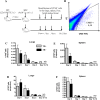
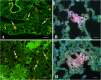
Mesenchymal stromal cells (MSC) have strong immunomodulatory properties and therefore can be used to control inflammation and tissue damage. It was suggested recently that MSC injections can be used to treat multi-drug resistant tuberculosis (TB). However, MSC trafficking and immunomodulatory effects of MSC injections during Mycobacterium tuberculosis (Mtb) infection have not been studied. To address this issue we have analyzed MSC distribution in tissues and local immunological effects of MSC injections in Mtb infected and uninfected mice. After intravenous injection, MSC accumulated preferentially in the lungs where they were located as cell aggregates in the alveolar walls. Immunological analysis of MSC effects included detection of activated, IFN-γ and IL-4 producing CD4+ lymphocytes, the frequency analysis of dendritic cells (CD11c+F4/80) and macrophages (CD11c-F4/80+) located in the lungs, the expression of IA/IE and CD11b molecules by these cells, and evaluation of 23 cytokines/chemokines in lung lysates. In the lungs of uninfected mice, MSC transfer markedly increased the percentage of IFN-γ+ CD4+ lymphocytes and dendritic cells, elevated levels of IA/IE expression by dendritic cells and macrophages, augmented local production of type 2 cytokines (IL-4, IL-5, IL-10) and chemokines (CCL2, CCL3, CCL4, CCL5, CXCL1), and downregulated type 1 and hematopoietic cytokines (IL-12p70, IFN-γ, IL-3, IL-6, GM-CSF). Compared to uninfected mice, Mtb infected mice had statistically higher "background" frequency of activated CD69+ and IFN-γ+ CD4+ lymphocytes and dendritic cells, and higher levels of cytokines in the lungs. The injections of MSC to Mtb infected mice did not show statistically significant effects on CD4+ lymphocytes, dendritic cells and macrophages, only slightly shifted cytokine profile, and did not change pathogen load or slow down TB progression. Lung section analysis showed that in Mtb infected mice, MSC could not be found in the proximity of the inflammatory foci. Thus, in healthy recipients, MSC administration dramatically changed T-cell function and cytokine/chemokine milieu in the lungs, most likely, due to capillary blockade. But, during Mtb infection, i.e., in the highly-inflammatory conditions, MSC did not affect T-cell function and the level of inflammation. The findings emphasize the importance of the evaluation of MSC effects locally at the site of their predominant post-injection localization and question MSC usefulness as anti-TB treatment.
Conflict of interest statement
Figures




Similar articles
-
CD11c(+) CD103(+) cells of Mycobacterium tuberculosis-infected C57BL/6 but not of BALB/c mice induce a high frequency of interferon-γ- or interleukin-17-producing CD4(+) cells.Immunology. 2015 Apr;144(4):574-86. doi: 10.1111/imm.12411. Immunology. 2015. PMID: 25322675 Free PMC article.
-
Interferon-gamma (IFN-gamma)-dependent protection and synthesis of chemoattractants for mononuclear leucocytes caused by IL-12 in the lungs of mice infected with Cryptococcus neoformans.Clin Exp Immunol. 1999 Jul;117(1):113-22. doi: 10.1046/j.1365-2249.1999.00955.x. Clin Exp Immunol. 1999. PMID: 10403924 Free PMC article.
-
Human IL-32 expression protects mice against a hypervirulent strain of Mycobacterium tuberculosis.Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):5111-6. doi: 10.1073/pnas.1424302112. Epub 2015 Mar 27. Proc Natl Acad Sci U S A. 2015. PMID: 25820174 Free PMC article.
-
Th22 response induced by Mycobacterium tuberculosis strains is closely related to severity of pulmonary lesions and bacillary load in patients with multi-drug-resistant tuberculosis.Clin Exp Immunol. 2021 Feb;203(2):267-280. doi: 10.1111/cei.13544. Epub 2020 Nov 18. Clin Exp Immunol. 2021. PMID: 33128773 Free PMC article. Review.
-
The role of IL-10 in immune regulation during M. tuberculosis infection.Mucosal Immunol. 2011 May;4(3):261-70. doi: 10.1038/mi.2011.7. Epub 2011 Mar 30. Mucosal Immunol. 2011. PMID: 21451501 Review.
Cited by
-
Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis.Front Cell Dev Biol. 2021 Jun 25;9:681171. doi: 10.3389/fcell.2021.681171. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249933 Free PMC article. Review.
-
Novel therapies using cell sheets engineered from allogeneic mesenchymal stem/stromal cells.Emerg Top Life Sci. 2020 Dec 17;4(6):677-689. doi: 10.1042/ETLS20200151. Emerg Top Life Sci. 2020. PMID: 33231260 Free PMC article. Review.
-
Mesenchymal stem cells protect against malaria pathogenesis by reprogramming erythropoiesis in the bone marrow.Cell Death Discov. 2020 Nov 15;6(1):125. doi: 10.1038/s41420-020-00363-2. Cell Death Discov. 2020. PMID: 33298881 Free PMC article.
-
Adipose-Derived Stem Cells from Systemic Sclerosis Patients Maintain Pro-Angiogenic and Antifibrotic Paracrine Effects In Vitro.J Clin Med. 2019 Nov 14;8(11):1979. doi: 10.3390/jcm8111979. J Clin Med. 2019. PMID: 31739569 Free PMC article.
-
Comparative Effects of Intra-Articular versus Intravenous Mesenchymal Stromal Cells Therapy in a Rat Model of Osteoarthritis by Destabilization of Medial Meniscus.Int J Mol Sci. 2023 Oct 24;24(21):15543. doi: 10.3390/ijms242115543. Int J Mol Sci. 2023. PMID: 37958526 Free PMC article.
References
-
- Najar M, Raicevic G, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy 2016; 18:160–71. doi: 10.1016/j.jcyt.2015.10.011 - DOI - PubMed
-
- Mattar P, Bieback K. Comparing the Immunomodulatory Properties of Bone Marrow, Adipose Tissue, and Birth-Associated Tissue Mesenchymal Stromal Cells. Front Immunol. 2015; 6:560 doi: 10.3389/fimmu.2015.00560 - DOI - PMC - PubMed
-
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838–43. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

