Measured pulmonary arterial tissue stiffness is highly sensitive to AFM indenter dimensions
- PMID: 28595103
- PMCID: PMC5582007
- DOI: 10.1016/j.jmbbm.2017.05.039
Measured pulmonary arterial tissue stiffness is highly sensitive to AFM indenter dimensions
Abstract
The mechanical properties of pulmonary tissues are important in normal function and the development of diseases such as pulmonary arterial hypertension. Hence it is critical to measure lung tissue micromechanical properties as accurately as possible in order to gain insight into the normal and pathological range of tissue stiffness associated with development, aging and disease processes. In this study, we used atomic force microscopy (AFM) micro-indentation to characterize the Young's modulus of small human pulmonary arteries (vessel diameter less than 100µm), and examined the influence of AFM tip geometry and diameter, lung tissue section thickness and the range of working force applied to the sample on the measured modulus. We observed a significant increase of the measured Young's modulus of pulmonary vessels (one order of magnitude) associated with the use of a pyramidal sharp AFM tips (20nm radius), compared to two larger spherical tips (1 and 2.5µm radius) which generated statistically indistinguishable results. The effect of tissue section thickness (ranging from 10 to 50 μm) on the measured elastic modulus was relatively smaller (<1-fold), but resulted in a significant increase in measured elastic modulus for the thinnest sections (10 μm) relative to the thicker (20 and 50 μm) sections. We also found that the measured elastic modulus depends modestly (again <1-fold), but significantly, on the magnitude of force applied, but only on thick (50 μm) and not thin (10 μm) tissue sections. Taken together these results demonstrate a dominant effect of indenter shape/radius on the measured elastic modulus of pulmonary arterial tissues, with lesser effects of tissue thickness and applied force. The results of this study highlight the importance of AFM parameter selection for accurate characterization of pulmonary arterial tissue mechanical properties, and allow for comparison of literature values for lung vessel tissue mechanical properties measured by AFM across a range of indenter and indentation parameters.
Keywords: AFM; Elasticity; Lung tissue; Micro-indentation; Pulmonary artery; Young's modulus.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
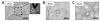


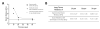
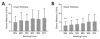
References
-
- Bilodeau GG. Regular pyramid punch problem. J Appl Mech. 1992;59:519–523. doi: 10.1115/1.2893754. - DOI
-
- Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

