Conversion to Parkinson Disease in the PARS Hyposmic and Dopamine Transporter-Deficit Prodromal Cohort
- PMID: 28595287
- PMCID: PMC5710321
- DOI: 10.1001/jamaneurol.2017.0985
Conversion to Parkinson Disease in the PARS Hyposmic and Dopamine Transporter-Deficit Prodromal Cohort
Abstract
Importance: Detecting individuals at risk for Parkinson disease (PD) during the prodromal phase could clarify disease mechanisms and allow for treatment earlier in the disease process to possibly slow or prevent the onset of motor PD.
Objective: To determine if the combination of smell identification testing followed by dopamine transporter (DAT) imaging can accurately and efficiently identify individuals from the general population at risk for conversion to a clinical diagnosis of PD.
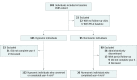
Design, setting, and participants: Participants were identified from the community by olfactory testing assessed longitudinally with DAT imaging 2 and 4 years after baseline and by annual clinical follow-up to determine whether they had clinical evidence to establish a PD diagnosis. Participants were contacted by mail and completed olfactory testing at home. Longitudinal follow-up of clinical measures and DAT imaging occurred at specialty centers. There were 203 hyposmic and 100 normosmic participants. A total of 185 hyposmic and 95 normosmic individuals had at least 1 follow-up visit, and 152 hyposmic participants (82.2%) were either observed for 4 years or converted to PD during follow-up.
Main outcomes and measures: Percentage of individuals with hyposmia and a DAT deficit that converted to PD and the change in PD clinical scale scores (Unified Parkinson's Disease Rating Scale) and DAT imaging during 4-year follow-up.
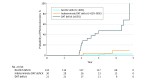
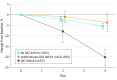
Results: Of 280 total participants, 140 (50.0%) were male, and the mean (SD) age of the cohort was 63 (8.7) years. Among 21 participants with hyposmia and a DAT deficit (65% or less of age-expected lowest putamen binding ratio) at baseline, 14 (67%) converted to PD at 4 years compared with 2 of 22 participants (9%) with a DAT in an indeterminate range (greater than 65%-80%) and 3 of 109 participants (2.8%) with no DAT deficit (greater than 80%) at baseline. Individuals with a baseline DAT deficit experienced a 4-year decline in DAT binding of 20.23% (SD, 15.04%) compared with 3.68% (SD, 18.36%) and 5.45% (SD, 13.58%) for participants with an indeterminate and no DAT deficit, respectively (P = .002). The relative risk of conversion to a diagnosis of PD in hyposmic individuals with a DAT deficit was 17.47 (95% CI, 7.02-43.45) compared with individuals with either indeterminate or no DAT deficit.
Conclusions and relevance: The combination of hyposmia and DAT deficit was highly predictive of conversion to PD within 4 years of clinical follow-up. Individuals with hyposmia and a DAT deficit had a 5% reduction in DAT binding annually, similar to early PD. These results provide a framework for planning disease prevention studies in PD.
Conflict of interest statement
Figures
Comment in
-
Dopaminergic Imaging and Prodromal Parkinson Disease: A Key Biomarker Arrives.JAMA Neurol. 2017 Aug 1;74(8):901-903. doi: 10.1001/jamaneurol.2017.0846. JAMA Neurol. 2017. PMID: 28595262 No abstract available.
-
Observations on a 2-Step Approach to Screening for Parkinson Disease-Reply.JAMA Neurol. 2017 Dec 1;74(12):1506-1507. doi: 10.1001/jamaneurol.2017.3208. JAMA Neurol. 2017. PMID: 29114721 No abstract available.
-
Observations on a 2-Step Approach to Screening for Parkinson Disease.JAMA Neurol. 2017 Dec 1;74(12):1506. doi: 10.1001/jamaneurol.2017.3202. JAMA Neurol. 2017. PMID: 29114724 No abstract available.
References
-
- Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600-1611. - PubMed
-
- Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28(3):311-318. - PubMed
-
- Streffer JR, Grachev ID, Fitzer-Attas C, et al. Prerequisites to launch neuroprotective trials in Parkinson’s disease: an industry perspective. Mov Disord. 2012;27(5):651-655. - PubMed
-
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38(8):1237-1244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

