Mitochondrial dysfunction underlies cognitive defects as a result of neural stem cell depletion and impaired neurogenesis
- PMID: 28595361
- PMCID: PMC5886206
- DOI: 10.1093/hmg/ddx217
Mitochondrial dysfunction underlies cognitive defects as a result of neural stem cell depletion and impaired neurogenesis
Abstract
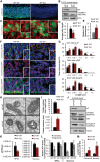
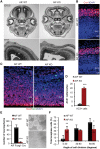
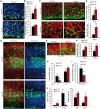
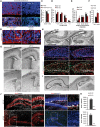
Mitochondrial dysfunction is a common feature of many genetic disorders that target the brain and cognition. However, the exact role these organelles play in the etiology of such disorders is not understood. Here, we show that mitochondrial dysfunction impairs brain development, depletes the adult neural stem cell (NSC) pool and impacts embryonic and adult neurogenesis. Using deletion of the mitochondrial oxidoreductase AIF as a genetic model of mitochondrial and neurodegenerative diseases revealed the importance of mitochondria in multiple steps of the neurogenic process. Developmentally, impaired mitochondrial function causes defects in NSC self-renewal, neural progenitor cell proliferation and cell cycle exit, as well as neuronal differentiation. Sustained mitochondrial dysfunction into adulthood leads to NSC depletion, loss of adult neurogenesis and manifests as a decline in brain function and cognitive impairment. These data demonstrate that mitochondrial dysfunction, as observed in genetic mitochondrial and neurodegenerative diseases, underlies the decline of brain function and cognition due to impaired stem cell maintenance and neurogenesis.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Khacho M., Slack R.S. (2015) Mitochondrial dynamics in neurodegeneration: from cell death to energetic states. AIMS Mol. Sci., 2, 161–174.
-
- Lin M.T., Beal M.F. (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 443, 787–795. - PubMed
-
- Khacho M., Clark A., Svoboda D.S., Azzi J., MacLaurin J.G., Meghaizel C., Sesaki H., Lagace D.C., Germain M., Harper M.E.. et al. (2016) Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell, 19, 232–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

