Albumin (BSA) adsorption onto graphite stepped surfaces
- PMID: 28595417
- PMCID: PMC5457296
- DOI: 10.1063/1.4984037
Albumin (BSA) adsorption onto graphite stepped surfaces
Abstract
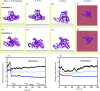
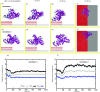
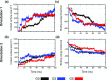
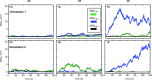
Nanomaterials are good candidates for the design of novel components with biomedical applications. For example, nano-patterned substrates may be used to immobilize protein molecules in order to integrate them in biosensing units. Here, we perform long MD simulations (up to 200 ns) using an explicit solvent and physiological ion concentrations to characterize the adsorption of bovine serum albumin (BSA) onto a nano-patterned graphite substrate. We have studied the effect of the orientation and step size on the protein adsorption and final conformation. Our results show that the protein is stable, with small changes in the protein secondary structure that are confined to the contact area and reveal the influence of nano-structuring on the spontaneous adsorption, protein-surface binding energies, and protein mobility. Although van der Waals (vdW) interactions play a dominant role, our simulations reveal the important role played by the hydrophobic lipid-binding sites of the BSA molecule in the adsorption process. The complex structure of these sites, that incorporate residues with different hydrophobic character, and their flexibility are crucial to understand the influence of the ion concentration and protein orientation in the different steps of the adsorption process. Our study provides useful information for the molecular engineering of components that require the immobilization of biomolecules and the preservation of their biological activity.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

