Omental adipose tissue is a more suitable source of canine Mesenchymal stem cells
- PMID: 28595579
- PMCID: PMC5465460
- DOI: 10.1186/s12917-017-1053-0
Omental adipose tissue is a more suitable source of canine Mesenchymal stem cells
Abstract
Background: Mesenchymal Stem Cells (MSCs) are a promising therapeutic tool in veterinary medicine. Currently the subcutaneous adipose tissue is the leading source of MSCs in dogs. MSCs derived from distinct fat depots have shown dissimilarities in their accessibility and therapeutic potential. The aims of our work were to determine the suitability of omental adipose tissue as a source of MSCs, according to sampling success, cell yield and paracrine properties of isolated cells, and compared to subcutaneous adipose tissue.
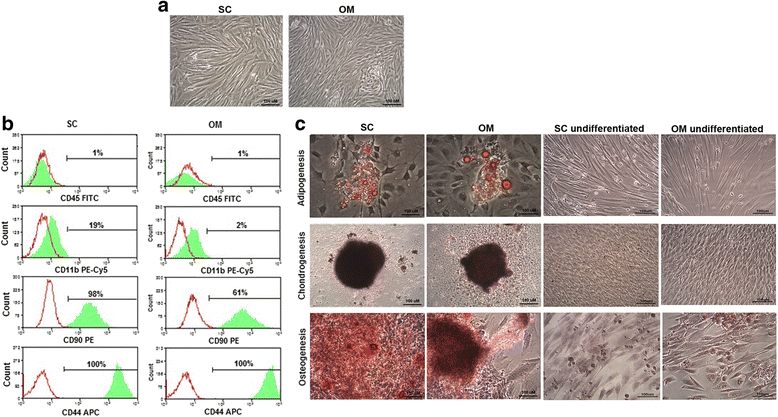
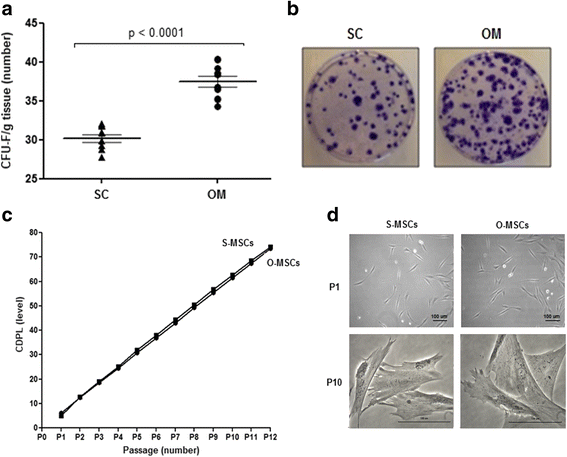
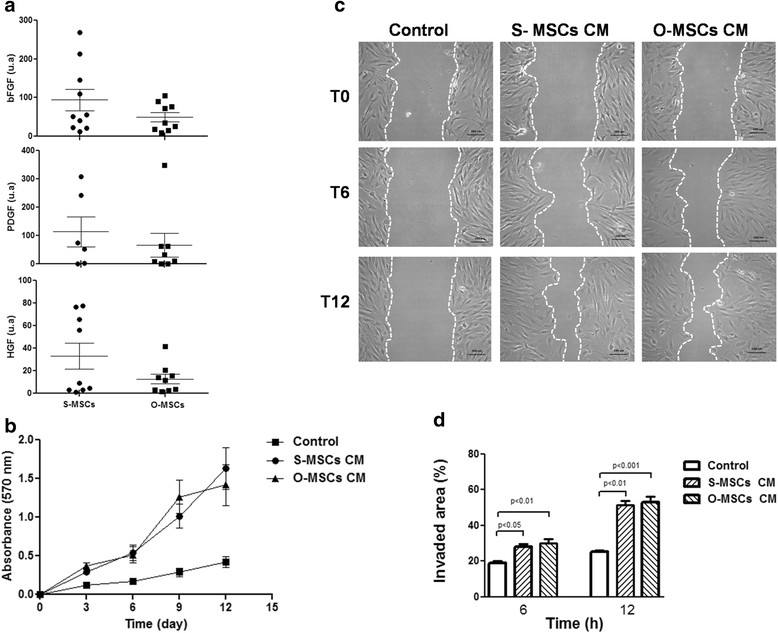
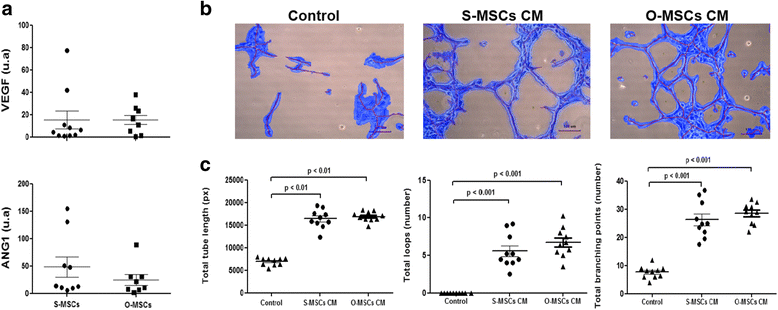
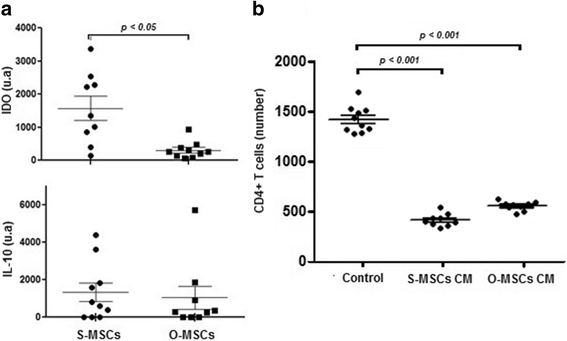
Results: While sampling success of omental adipose tissue was 100% (14 collections from14 donors) for subcutaneous adipose tissue it was 71% (10 collections from 14 donors). MSCs could be isolated from both sources. Cell yield was significantly higher for omental than for subcutaneous adipose tissue (38 ± 1 vs. 30 ± 1 CFU-F/g tissue, p < 0.0001). No differences were observed between sources regarding cell proliferation potential (73 ± 1 vs. 74 ± 1 CDPL) and cell senescence (at passage 10, both cultures presented enlarged cells with cytoplasmic vacuoles and cellular debris). Omental- and subcutaneous-derived MSCs expressed at the same level bFGF, PDGF, HGF, VEGF, ANG1 and IL-10. Irrespective of the source, isolated MSCs induced proliferation, migration and vascularization of target cells, and inhibited the activation of T lymphocytes.
Conclusion: Compared to subcutaneous adipose tissue, omental adipose tissue is a more suitable source of MSCs in dogs. Since it can be procured from donors with any body condition, its collection procedure is always feasible, its cell yield is high and the MSCs isolated from it have desirable differentiation and paracrine potentials.
Keywords: Adipose tissue; Canine; Dog; Mesenchymal stem cell; Omentum; Source.
Figures
Similar articles
-
Comparison of Stromal/Stem Cells Isolated from Human Omental and Subcutaneous Adipose Depots: Differentiation and Immunophenotypic Characterization.Cells Tissues Organs. 2014;200(3-4):204-11. doi: 10.1159/000430088. Epub 2015 Jun 18. Cells Tissues Organs. 2014. PMID: 26089088
-
Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects.Metabolism. 2004 May;53(5):632-7. doi: 10.1016/j.metabol.2003.11.012. Metabolism. 2004. PMID: 15131769
-
Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources.BMC Vet Res. 2021 Dec 18;17(1):388. doi: 10.1186/s12917-021-03100-8. BMC Vet Res. 2021. PMID: 34922529 Free PMC article.
-
Current Status on Canine Foetal Fluid and Adnexa Derived Mesenchymal Stem Cells.Animals (Basel). 2021 Jul 30;11(8):2254. doi: 10.3390/ani11082254. Animals (Basel). 2021. PMID: 34438710 Free PMC article. Review.
-
The Omentum-A Forgotten Structure in Veterinary Surgery in Small Animals' Surgery.Animals (Basel). 2024 Jun 21;14(13):1848. doi: 10.3390/ani14131848. Animals (Basel). 2024. PMID: 38997960 Free PMC article. Review.
Cited by
-
<Editors' Choice> Supernatant from activated omentum accelerates wound healing in diabetic mice wound model.Nagoya J Med Sci. 2023 Aug;85(3):528-541. doi: 10.18999/nagjms.85.3.528. Nagoya J Med Sci. 2023. PMID: 37829482 Free PMC article.
-
Intra-arterial renal infusion of autologous mesenchymal stem cells for treatment of chronic kidney disease in cats: Phase I clinical trial.J Vet Intern Med. 2019 May;33(3):1353-1361. doi: 10.1111/jvim.15486. Epub 2019 Mar 29. J Vet Intern Med. 2019. PMID: 30924554 Free PMC article.
-
Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation.Biomol Ther (Seoul). 2019 Jan 1;27(1):25-33. doi: 10.4062/biomolther.2017.260. Biomol Ther (Seoul). 2019. PMID: 29902862 Free PMC article. Review.
-
Comparison of Sources and Methods for the Isolation of Equine Adipose Tissue-Derived Stromal/Stem Cells and Preliminary Results on Their Reaction to Incubation with 5-Azacytidine.Animals (Basel). 2022 Aug 11;12(16):2049. doi: 10.3390/ani12162049. Animals (Basel). 2022. PMID: 36009640 Free PMC article.
-
An Outstanding Role of Adipose Tissue in Canine Stem Cell Therapy.Animals (Basel). 2022 Apr 22;12(9):1088. doi: 10.3390/ani12091088. Animals (Basel). 2022. PMID: 35565514 Free PMC article. Review.
References
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

