The efficacy and safety of Danggui-Sayuk-Ga-Osuyu-Saenggang-tang on Korean patients with cold hypersensitivity in the hands: study protocol for a pilot, double-blind, randomized, placebo-controlled, parallel-group clinical trial
- PMID: 28595610
- PMCID: PMC5465469
- DOI: 10.1186/s13063-017-2002-8
The efficacy and safety of Danggui-Sayuk-Ga-Osuyu-Saenggang-tang on Korean patients with cold hypersensitivity in the hands: study protocol for a pilot, double-blind, randomized, placebo-controlled, parallel-group clinical trial
Abstract
Background: In recent years, cold hypersensitivity in the hands (CHH) has become a common ailment of women in Korea. It can lead to gynecological problems such as irregular menstruation, miscarriage, and infertility. Traditionally, Korean herbal medicine has been the primary treatment method used to balance thermoregulation in the human body; however, its effectiveness has not been confirmed through systematic study. Thus, in this trial, we will investigate the feasibility of a full randomized clinical trial, Danggui-Sayuk-Ga-Osuyu-Saenggang-tang (DSGOST) in Korean women with CHH.
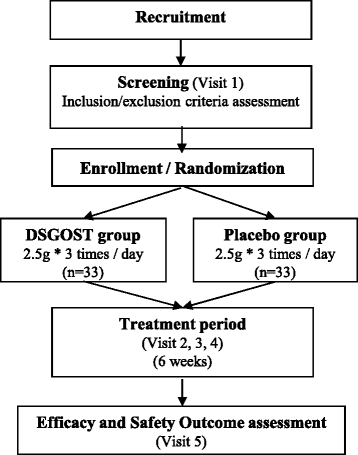
Methods: This study will be a pilot, multicenter, double-blind, randomized, parallel-group, two-arm, placebo-controlled clinical trial. A total of 66 participants will be randomly divided into two groups, a DSGOST treatment group and a placebo control group, in a 1:1 ratio using a web-based randomization system. Each group will take DSGOST or placebo three times daily for 6 weeks. The primary outcome will be measured using Visual Analogue Scale (VAS) scores of CHH. Secondary outcomes will include changes in skin temperature of the hands, Clinical Global Impressions (CGI) scale scores, recovery rate of skin temperature of the hands after the cold stress test, and the Korean version of the WHO Quality of Life Scale, abbreviated version (WHOQOL-BREF).
Discussion: This trial will be the first trial to reflect the newly defined disease range of CHH which was compiled by Korean medicine expert consensus. This study will provide considerable evidence for further large-scale trials and general clinical guidelines for CHH in the Korean medical field.
Trial registration: This study is registered at ClinicalTrials.gov, ID: NCT02645916 . Registered on 30 December 2015.
Keywords: Cold hypersensitivity; Cold temperature; Herbal medicine; Randomized clinical trial.
Figures
Similar articles
-
Efficacy and safety of Ojeok-san in Korean female patients with cold hypersensitivity in the hands and feet: study protocol for a randomized, double-blinded, placebo-controlled, multicenter pilot study.Trials. 2018 Nov 29;19(1):662. doi: 10.1186/s13063-018-3013-9. Trials. 2018. PMID: 30497488 Free PMC article.
-
The efficacy and safety of Sipjeondaebo-tang in Korean patients with cold hypersensitivity in the hands and feet: a protocol for a pilot, randomized, double-blind, placebo-controlled, parallel-group clinical trial.Trials. 2019 Apr 15;20(1):217. doi: 10.1186/s13063-019-3286-7. Trials. 2019. PMID: 30987667 Free PMC article.
-
Efficacy and safety of Onkyeong-tang in treating cold hypersensitivity in the feet of Korean women: protocol for a double-blind, randomized, placebo-controlled, parallel-group, multicenter clinical study.Trials. 2020 May 18;21(1):410. doi: 10.1186/s13063-020-04265-7. Trials. 2020. PMID: 32423429 Free PMC article.
-
Herbal Medicines for Cold Hypersensitivity in the Hands and Feet: A Systematic Review and Meta-Analysis.J Altern Complement Med. 2018 Dec;24(12):1150-1158. doi: 10.1089/acm.2018.0009. Epub 2018 Jul 11. J Altern Complement Med. 2018. PMID: 29993255 Free PMC article.
-
Regulation and status of herbal medicine clinical trials in Korea: a narrative review.Integr Med Res. 2021 Jun;10(2):100688. doi: 10.1016/j.imr.2020.100688. Epub 2020 Nov 4. Integr Med Res. 2021. PMID: 33717976 Free PMC article. Review.
Cited by
-
Study Protocol for Multi-Center, Open-Label, Randomized Controlled Trial for Assessing the Efficacy and Safety of Electroacupuncture for Cold Hypersensitivity in Hands and Feet.J Pharmacopuncture. 2025 Mar 31;28(1):47-56. doi: 10.3831/KPI.2025.28.1.47. J Pharmacopuncture. 2025. PMID: 40165876 Free PMC article.
-
DSGOST regulates resistance via activation of autophagy in gastric cancer.Cell Death Dis. 2018 May 29;9(6):649. doi: 10.1038/s41419-018-0658-y. Cell Death Dis. 2018. PMID: 29844404 Free PMC article.
-
Efficacy and safety of Ojeok-san in Korean female patients with cold hypersensitivity in the hands and feet: study protocol for a randomized, double-blinded, placebo-controlled, multicenter pilot study.Trials. 2018 Nov 29;19(1):662. doi: 10.1186/s13063-018-3013-9. Trials. 2018. PMID: 30497488 Free PMC article.
-
The definition and diagnosis of cold hypersensitivity in the hands and feet: Finding from the experts survey.Integr Med Res. 2018 Mar;7(1):61-67. doi: 10.1016/j.imr.2017.11.001. Epub 2017 Nov 16. Integr Med Res. 2018. PMID: 29629292 Free PMC article.
-
Efficacy and safety of ucha-shinki-hwan on korean patients with cold hypersensitivity in the hands and feet: Study protocol clinical trial (SPIRIT Compliant).Medicine (Baltimore). 2020 Feb;99(8):e19110. doi: 10.1097/MD.0000000000019110. Medicine (Baltimore). 2020. PMID: 32080084 Free PMC article. Clinical Trial.
References
-
- Sun SH, Go HY, Ko SW, Cho YY, Shin JH, Kim TH, et al. A Delphi study for treatment standardization of coldness in hands and Feet. J Soc Preven Korean Med. 2015;19(3):131–140.
-
- Lee SL, Lee KS, Song BK. The literature review on the cold hypersensitivity of women. Korean J Obstet Gynecol. 1996;9:55–80.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical