Developing and evaluating multimedia information resources to improve engagement of children, adolescents, and their parents with trials (TRECA study): Study protocol for a series of linked randomised controlled trials
- PMID: 28595613
- PMCID: PMC5465557
- DOI: 10.1186/s13063-017-1962-z
Developing and evaluating multimedia information resources to improve engagement of children, adolescents, and their parents with trials (TRECA study): Study protocol for a series of linked randomised controlled trials
Abstract
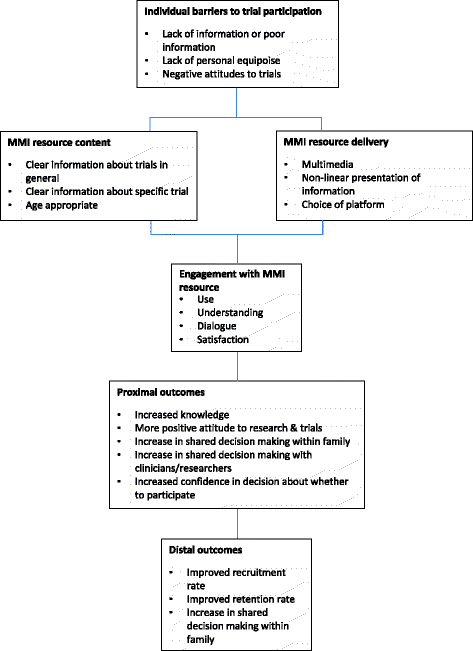
Background: Randomised controlled trials are widely established as the best method for testing health interventions whilst minimising bias. However, recruitment and subsequent retention of children and adolescents in healthcare trials is challenging. Participant information sheets are often lengthy and difficult to read and understand. Presenting key information using multimedia may help to overcome these limitations and better support young people and their parents in deciding whether to participate in a clinical trial.
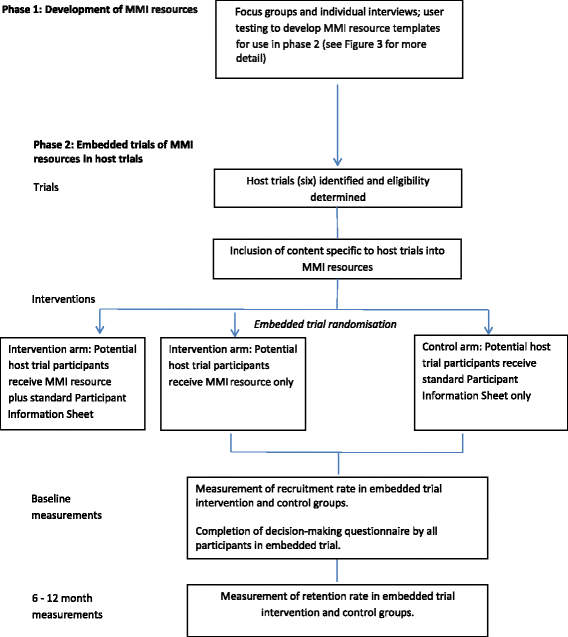
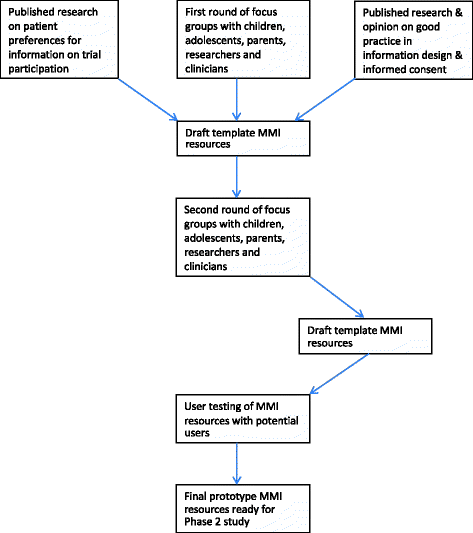
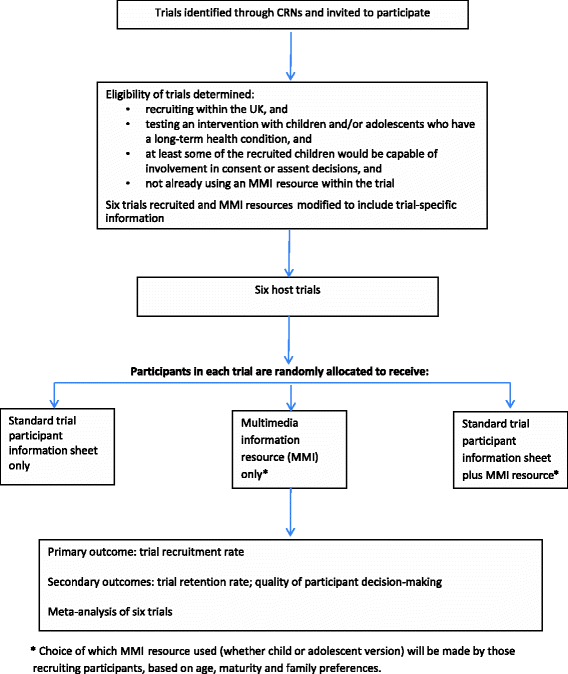
Methods: The TRECA (TRials Engagement in Children and Adolescents) study has two phases. The first phase involves a qualitative study with children and adolescents and their parents to inform the development of multimedia information resources and iterative user testing to refine the resources. The second phase will embed the use of the multimedia information resources into six host trials in the United Kingdom. Patients and parents approached to participate in the host trials will be randomly allocated to either use the multimedia information resource in conjunction with standard participant information sheets, the multimedia information resource alone, or the standard participant information sheets alone. The primary outcome will be the effect of the multimedia information resources on recruitment into trials. Other outcomes measured include the effect of multimedia information resources on retention of participants into the host trials and the impact on family members' decision-making processes, when compared to standard participant information sheets alone.
Discussion: This study will inform whether multimedia information resources, when developed using participatory design principles, are able to increase recruitment and retention of children and adolescents into trials. There is also the potential for patients to make better informed decisions through the use of multimedia information resources. The multimedia information resources also have the potential to assist with providing information on other healthcare decisions outside of clinical trials.
Trial registration: ISRCTN registry: ISRCTN73136092 (doi: 10.1186/ISRCTN73136092 ). Registered on 24 August 2016.
Keywords: Adolescent; Child; Consent; Decision-making; Information; Intervention; Multimedia; Parent; Recruitment; Retention; Trial participation.
Figures
Similar articles
-
The effectiveness and acceptability of multimedia information when recruiting children and young people to trials: the TRECA meta-analysis of SWATs.Health Soc Care Deliv Res. 2023 Nov;11(24):1-112. doi: 10.3310/HTPM3841. Health Soc Care Deliv Res. 2023. PMID: 38140894
-
Supporting children and young people when making decisions about joining clinical trials: qualitative study to inform multimedia website development.BMJ Open. 2019 Jan 9;9(1):e023984. doi: 10.1136/bmjopen-2018-023984. BMJ Open. 2019. PMID: 30782720 Free PMC article.
-
Improving recruitment to a study of telehealth management for COPD: a cluster randomised controlled 'study within a trial' (SWAT) of a multimedia information resource.Trials. 2019 Jul 24;20(1):453. doi: 10.1186/s13063-019-3496-z. Trials. 2019. PMID: 31340853 Free PMC article. Clinical Trial.
-
Factors that impact on recruitment to randomised trials in health care: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2020 Oct 7;10(10):MR000045. doi: 10.1002/14651858.MR000045.pub2. Cochrane Database Syst Rev. 2020. PMID: 33026107 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Feasibility of a new 'balanced binocular viewing' treatment for unilateral amblyopia in children aged 3-8 years (BALANCE): results of a phase 2a randomised controlled feasibility trial.BMJ Open. 2024 Jul 30;14(7):e082472. doi: 10.1136/bmjopen-2023-082472. BMJ Open. 2024. PMID: 39079927 Free PMC article. Clinical Trial.
-
Can we achieve better trial recruitment by presenting patient information through multimedia? Meta-analysis of 'studies within a trial' (SWATs).BMC Med. 2023 Nov 8;21(1):425. doi: 10.1186/s12916-023-03081-5. BMC Med. 2023. PMID: 37940944 Free PMC article.
-
The COMBAT Project: study protocol for the development of a core outcome set for morbidity following surgery in paediatric brain tumour patients.Trials. 2025 Aug 11;26(1):286. doi: 10.1186/s13063-025-09004-4. Trials. 2025. PMID: 40790773 Free PMC article.
-
Offer of a bandage versus rigid immobilisation in 4- to 15-year-olds with distal radius torus fractures: the FORCE equivalence RCT.Health Technol Assess. 2022 Jul;26(33):1-78. doi: 10.3310/BDNS6122. Health Technol Assess. 2022. PMID: 35904496 Free PMC article. Clinical Trial.
-
Undertaking Studies Within A Trial to evaluate recruitment and retention strategies for randomised controlled trials: lessons learnt from the PROMETHEUS research programme.Health Technol Assess. 2024 Jan;28(2):1-114. doi: 10.3310/HTQW3107. Health Technol Assess. 2024. PMID: 38327177 Free PMC article.
References
-
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrom M, Johansen M, Taskila TK, Sullivan FM, Wilson S, Jackson C, et al. Methods to improve recruitment to randomised controlled trials: cochrane systematic review and meta-analysis. BMJ Open 2013;3(2). http://bmjopen.bmj.com/content/3/2/e002360 - PMC - PubMed
-
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, Elbourne DR, Francis D, Garcia J, Roberts I, Snowdon C. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical