The evolution of trypanosomatid taxonomy
- PMID: 28595622
- PMCID: PMC5463341
- DOI: 10.1186/s13071-017-2204-7
The evolution of trypanosomatid taxonomy
Abstract
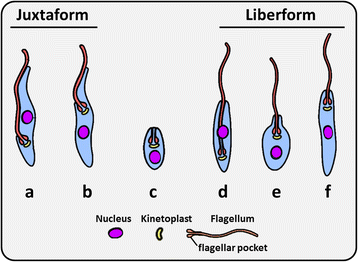
Trypanosomatids are protozoan parasites of the class Kinetoplastida predominately restricted to invertebrate hosts (i.e. possess a monoxenous life-cycle). However, several genera are pathogenic to humans, animals and plants, and have an invertebrate vector that facilitates their transmission (i.e. possess a dixenous life-cycle). Phytomonas is one dixenous genus that includes several plant pathogens transmitted by phytophagous insects. Trypanosoma and Leishmania are dixenous genera that infect vertebrates, including humans, and are transmitted by hematophagous invertebrates. Traditionally, monoxenous trypanosomatids such as Leptomonas were distinguished from morphologically similar dixenous species based on their restriction to an invertebrate host. Nonetheless, this criterion is somewhat flawed as exemplified by Leptomonas seymouri which reportedly infects vertebrates opportunistically. Similarly, Novymonas and Zelonia are presumably monoxenous genera yet sit comfortably in the dixenous clade occupied by Leishmania. The isolation of Leishmania macropodum from a biting midge (Forcipomyia spp.) rather than a phlebotomine sand fly calls into question the exclusivity of the Leishmania-sand fly relationship, and its suitability for defining the Leishmania genus. It is now accepted that classic genus-defining characteristics based on parasite morphology and host range are insufficient to form the sole basis of trypanosomatid taxonomy as this has led to several instances of paraphyly. While improvements have been made, resolution of evolutionary relationships within the Trypanosomatidae is confounded by our incomplete knowledge of its true diversity. The known trypanosomatids probably represent a fraction of those that exist and isolation of new species will help resolve relationships in this group with greater accuracy. This review incites a dialogue on how our understanding of the relationships between certain trypanosomatids has shifted, and discusses new knowledge that informs the present taxonomy of these important parasites.
Keywords: Leishmania; Leptomonas; Phylogenetics; Systematics; Taxonomy; Trypanosomatid; Zelonia.
Figures
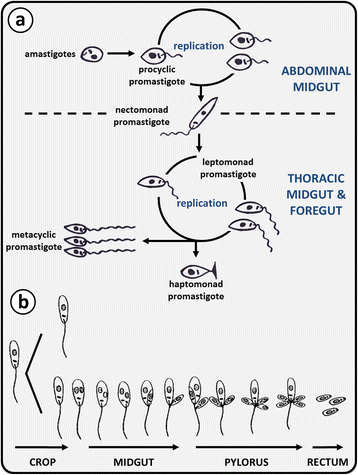
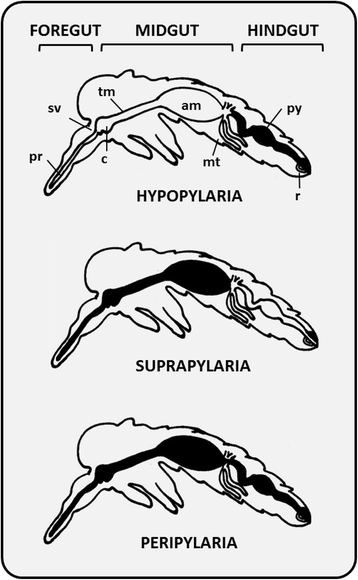
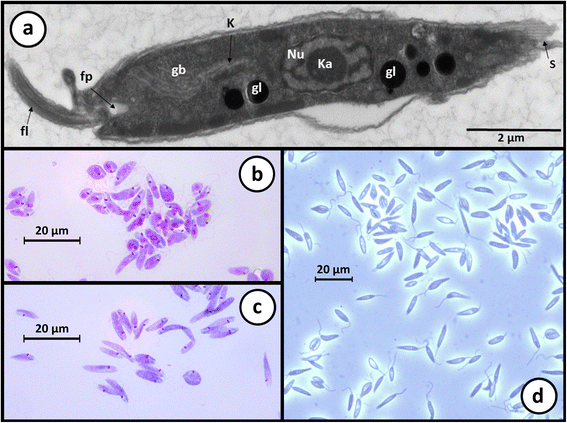
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

