Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson's disease: Relevance to gene and environment interactions in metal neurotoxicity
- PMID: 28595911
- PMCID: PMC5736468
- DOI: 10.1016/j.neuro.2017.06.002
Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson's disease: Relevance to gene and environment interactions in metal neurotoxicity
Abstract
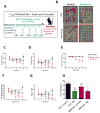
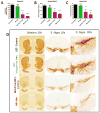
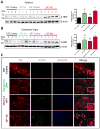
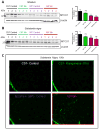
Parkinson's disease (PD) is now recognized as a neurodegenerative condition caused by a complex interplay of genetic and environmental influences. Chronic manganese (Mn) exposure has been implicated in the development of PD. Since mitochondrial dysfunction is associated with PD pathology as well as Mn neurotoxicity, we investigated whether Mn exposure augments mitochondrial dysfunction and neurodegeneration in the nigrostriatal dopaminergic system using a newly available mitochondrially defective transgenic mouse model of PD, the MitoPark mouse. This unique PD model recapitulates key features of the disease including progressive neurobehavioral changes and neuronal degeneration. We exposed MitoPark mice to a low dose of Mn (10mg/kg, p.o.) daily for 4 weeks starting at age 8 wks and then determined the behavioral, neurochemical and histological changes. Mn exposure accelerated the rate of progression of motor deficits in MitoPark mice when compared to the untreated MitoPark group. Mn also worsened olfactory function in this model. Most importantly, Mn exposure intensified the depletion of striatal dopamine and nigral TH neuronal loss in MitoPark mice. The neurodegenerative changes were accompanied by enhanced oxidative damage in the striatum and substantia nigra (SN) of MitoPark mice treated with Mn. Furthermore, Mn-treated MitoPark mice had significantly more oligomeric protein and IBA-1-immunoreactive microglia cells, suggesting Mn augments neuroinflammatory processes in the nigrostriatal pathway. To further confirm the direct effect of Mn on impaired mitochondrial function, we also generated a mitochondrially defective dopaminergic cell model by knocking out the TFAM transcription factor by using a CRISPR-Cas9 gene-editing method. Seahorse mitochondrial bioenergetic analysis revealed that Mn decreases mitochondrial basal and ATP-linked respiration in the TFAM KO cells. Collectively, our results reveal that Mn can augment mitochondrial dysfunction to exacerbate nigrostriatal neurodegeneration and PD-related behavioral symptoms. Our study also demonstrates that the MitoPark mouse is an excellent model to study the gene-environment interactions associated with mitochondrial defects in the nigral dopaminergic system as well as to evaluate the contribution of potential environmental toxicant interactions in a slowly progressive model of Parkinsonism.
Keywords: Animal model; Dopamine; Gene-environment interaction; Manganese; MitoPark; Mitochondria; Neuroinflammation; Parkinson’s disease; TFAM.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
Dopaminergic Neurons Exhibit an Age-Dependent Decline in Electrophysiological Parameters in the MitoPark Mouse Model of Parkinson's Disease.J Neurosci. 2016 Apr 6;36(14):4026-37. doi: 10.1523/JNEUROSCI.1395-15.2016. J Neurosci. 2016. PMID: 27053209 Free PMC article.
-
Mito-Apocynin Prevents Mitochondrial Dysfunction, Microglial Activation, Oxidative Damage, and Progressive Neurodegeneration in MitoPark Transgenic Mice.Antioxid Redox Signal. 2017 Nov 10;27(14):1048-1066. doi: 10.1089/ars.2016.6905. Epub 2017 Apr 4. Antioxid Redox Signal. 2017. PMID: 28375739 Free PMC article.
-
Progressive parkinsonism due to mitochondrial impairment: Lessons from the MitoPark mouse model.Exp Neurol. 2021 Jul;341:113707. doi: 10.1016/j.expneurol.2021.113707. Epub 2021 Mar 20. Exp Neurol. 2021. PMID: 33753138 Free PMC article. Review.
-
Characterization of nonmotor behavioral impairments and their neurochemical mechanisms in the MitoPark mouse model of progressive neurodegeneration in Parkinson's disease.Exp Neurol. 2021 Jul;341:113716. doi: 10.1016/j.expneurol.2021.113716. Epub 2021 Apr 8. Exp Neurol. 2021. PMID: 33839143 Free PMC article.
-
Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review.
Cited by
-
Elevated circulating magnesium levels in patients with Parkinson's disease: a meta-analysis.Neuropsychiatr Dis Treat. 2018 Nov 19;14:3159-3168. doi: 10.2147/NDT.S186209. eCollection 2018. Neuropsychiatr Dis Treat. 2018. PMID: 30510425 Free PMC article.
-
Environmental neurotoxicant-induced dopaminergic neurodegeneration: a potential link to impaired neuroinflammatory mechanisms.Pharmacol Ther. 2019 May;197:61-82. doi: 10.1016/j.pharmthera.2019.01.001. Epub 2019 Jan 22. Pharmacol Ther. 2019. PMID: 30677475 Free PMC article. Review.
-
Parkinson's Disease and the Gut: Future Perspectives for Early Diagnosis.Front Neurosci. 2020 Jun 17;14:626. doi: 10.3389/fnins.2020.00626. eCollection 2020. Front Neurosci. 2020. PMID: 32625058 Free PMC article. Review.
-
Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation.Antioxidants (Basel). 2022 Feb 17;11(2):408. doi: 10.3390/antiox11020408. Antioxidants (Basel). 2022. PMID: 35204290 Free PMC article. Review.
-
Next Generation Precision Medicine: CRISPR-mediated Genome Editing for the Treatment of Neurodegenerative Disorders.J Neuroimmune Pharmacol. 2019 Dec;14(4):608-641. doi: 10.1007/s11481-019-09849-y. Epub 2019 Apr 23. J Neuroimmune Pharmacol. 2019. PMID: 31011884 Free PMC article. Review.
References
-
- Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free radical biology & medicine. 2013;60:282–291. - PMC - PubMed
-
- Anantharam V, Kitazawa M, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Blockade of PKCdelta proteolytic activation by loss of function mutants rescues mesencephalic dopaminergic neurons from methylcyclopentadienyl manganese tricarbonyl (MMT)-induced apoptotic cell death. Annals of the New York Academy of Sciences. 2004;1035:271–289. - PubMed
-
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(5):1738–1751. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

