HPV8 Field Cancerization in a Transgenic Mouse Model Is due to Lrig1+ Keratinocyte Stem Cell Expansion
- PMID: 28595997
- PMCID: PMC5613749
- DOI: 10.1016/j.jid.2017.04.039
HPV8 Field Cancerization in a Transgenic Mouse Model Is due to Lrig1+ Keratinocyte Stem Cell Expansion
Abstract
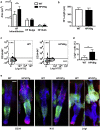
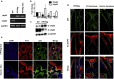
β-Human papillomaviruses (HPVs) cause near ubiquitous latent skin infection within long-lived hair follicle (HF) keratinocyte stem cells. In patients with epidermodysplasia verruciformis, β-HPV viral replication is associated with skin keratosis and cutaneous squamous cell carcinoma. To determine the role of HF keratinocyte stem cells in β-HPV-induced skin carcinogenesis, we utilized a transgenic mouse model in which the keratin 14 promoter drives expression of the entire HPV8 early region (HPV8tg). HPV8tg mice developed thicker skin in comparison with wild-type littermates consistent with a hyperproliferative epidermis. HF keratinocyte proliferation was evident within the Lrig1+ keratinocyte stem cell population (69 vs. 55%, P < 0.01, n = 7), and not in the CD34+, LGR5+, and LGR6+ keratinocyte stem cell populations. This was associated with a 2.8-fold expansion in Lrig1+ keratinocytes and 3.8-fold increased colony-forming efficiency. Consistent with this, we observed nuclear p63 expression throughout this population and the HF infundibulum and adjoining interfollicular epidermis, associated with a switch from p63 transcriptional activation isoforms to ΔNp63 isoforms in HPV8tg skin. Epidermodysplasia verruciformis keratosis and in some cases actinic keratoses demonstrated similar histology associated with β-HPV reactivation and nuclear p63 expression within the HF infundibulum and perifollicular epidermis. These findings would suggest that β-HPV field cancerization arises from the HF junctional zone and predispose to squamous cell carcinoma.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
HPV8-induced STAT3 activation led keratinocyte stem cell expansion in human actinic keratoses.JCI Insight. 2024 Jun 25;9(15):e177898. doi: 10.1172/jci.insight.177898. JCI Insight. 2024. PMID: 38916963 Free PMC article.
-
E6 and E7 from beta HPV38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice.PLoS Pathog. 2011 Jul;7(7):e1002125. doi: 10.1371/journal.ppat.1002125. Epub 2011 Jul 14. PLoS Pathog. 2011. PMID: 21779166 Free PMC article.
-
Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis.Cell Stem Cell. 2009 May 8;4(5):427-39. doi: 10.1016/j.stem.2009.04.014. Cell Stem Cell. 2009. PMID: 19427292 Free PMC article.
-
Keratinocyte stem cells and the targets for nonmelanoma skin cancer.Photochem Photobiol. 2012 Sep-Oct;88(5):1099-110. doi: 10.1111/j.1751-1097.2012.01079.x. Epub 2012 Jan 31. Photochem Photobiol. 2012. PMID: 22211846 Free PMC article. Review.
-
Human Papillomaviruses and Skin Cancer.Adv Exp Med Biol. 2020;1268:195-209. doi: 10.1007/978-3-030-46227-7_10. Adv Exp Med Biol. 2020. PMID: 32918220 Review.
Cited by
-
The Protein Tyrosine Phosphatase H1 PTPH1 Supports Proliferation of Keratinocytes and is a Target of the Human Papillomavirus Type 8 E6 Oncogene.Cells. 2019 Mar 14;8(3):244. doi: 10.3390/cells8030244. Cells. 2019. PMID: 30875834 Free PMC article.
-
Cutaneous Papillomaviruses and Non-melanoma Skin Cancer: Causal Agents or Innocent Bystanders?Front Microbiol. 2018 May 2;9:874. doi: 10.3389/fmicb.2018.00874. eCollection 2018. Front Microbiol. 2018. PMID: 29770129 Free PMC article. Review.
-
Changing Stem Cell Dynamics during Papillomavirus Infection: Potential Roles for Cellular Plasticity in the Viral Lifecycle and Disease.Viruses. 2017 Aug 12;9(8):221. doi: 10.3390/v9080221. Viruses. 2017. PMID: 28805675 Free PMC article. Review.
-
Beta Human Papillomavirus 8 E6 Induces Micronucleus Formation and Promotes Chromothripsis.J Virol. 2022 Oct 12;96(19):e0101522. doi: 10.1128/jvi.01015-22. Epub 2022 Sep 21. J Virol. 2022. PMID: 36129261 Free PMC article.
-
Cancer stem cells in esophageal squamous cell cancer.Oncol Lett. 2019 Nov;18(5):5022-5032. doi: 10.3892/ol.2019.10900. Epub 2019 Sep 20. Oncol Lett. 2019. PMID: 31612013 Free PMC article. Review.
References
-
- Abbas O., Richards J.E., Yaar R., Mahalingam M. Stem cell markers (cytokeratin 15, cytokeratin 19 and p63) in in situ and invasive cutaneous epithelial lesions. Mod Pathol. 2011;24:90–97. - PubMed
-
- Azzimonti B., Mondini M., De Andrea M., Gioia D., Dianzani U., Mesturini R. CD8+ T-cell lymphocytopenia and lack of EVER mutations in a patient with clinically and virologically typical epidermodysplasia verruciformis. Arch Dermatol. 2005;141:1323–1325. - PubMed
-
- Banerjee M., Sarma N., Biswas R., Roy J., Mukherjee A., Giri A.K. DNA repair deficiency leads to susceptibility to develop arsenic-induced premalignant skin lesions. Int J Cancer. 2008;123:283–287. - PubMed
-
- Borgogna C., Landini M.M., Lanfredini S., Doorbar J., Bouwes Bavinck J.N., Quint K.D. Characterization of skin lesions induced by skin-tropic α- and β-papillomaviruses in a patient with epidermodysplasia verruciformis. Br J Dermatol. 2014;171:1550–1554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

