Successful Repeated Hepatic Gene Delivery in Mice and Non-human Primates Achieved by Sequential Administration of AAV5ch and AAV1
- PMID: 28596114
- PMCID: PMC5542767
- DOI: 10.1016/j.ymthe.2017.05.003
Successful Repeated Hepatic Gene Delivery in Mice and Non-human Primates Achieved by Sequential Administration of AAV5ch and AAV1
Abstract
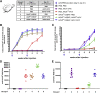
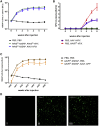
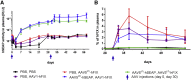
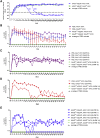
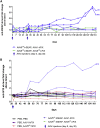
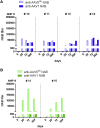
In the gene therapy field, re-administration of adeno-associated virus (AAV) is an important topic because a decrease in therapeutic protein expression might occur over time. However, an efficient re-administration with the same AAV serotype is impossible due to serotype-specific, anti-AAV neutralizing antibodies (NABs) that are produced after initial AAV treatment. To address this issue, we explored the feasibility of using chimeric AAV serotype 5 (AAV5ch) and AAV1 for repeated liver-targeted gene delivery. To develop a relevant model, we immunized animals with a high dose of AAV5ch-human secreted embryonic alkaline phosphatase (hSEAP) that generates high levels of anti-AAV5ch NAB. Secondary liver transduction with the same dose of AAV1-human factor IX (hFIX) in the presence of high levels of anti-AAV5ch NAB proved to be successful because expression/activity of both reporter transgenes was observed. This is the first time that two different transgenes are shown to be produced by non-human primate (NHP) liver after sequential administration of clinically relevant doses of both AAV5ch and AAV1. The levels of transgene proteins achieved after delivery with AAV5ch and AAV1 illustrate the possibility of both serotypes for liver targeting. Furthermore, transgene DNA and RNA biodistribution patterns provided insight into the potential cause of decrease or loss of transgene protein expression over time in NHPs.
Keywords: AAV; gene therapy; re-administration of gene therapy.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Immunoadsorption enables successful rAAV5-mediated repeated hepatic gene delivery in nonhuman primates.Blood Adv. 2019 Sep 10;3(17):2632-2641. doi: 10.1182/bloodadvances.2019000380. Blood Adv. 2019. PMID: 31501158 Free PMC article.
-
Effect of viral dose on neutralizing antibody response and transgene expression after AAV1 vector re-administration in mice.Gene Ther. 2008 Jan;15(1):54-60. doi: 10.1038/sj.gt.3303037. Epub 2007 Oct 25. Gene Ther. 2008. PMID: 17960164
-
Long-Term Follow-Up of the First in Human Intravascular Delivery of AAV for Gene Transfer: AAV2-hFIX16 for Severe Hemophilia B.Mol Ther. 2020 Sep 2;28(9):2073-2082. doi: 10.1016/j.ymthe.2020.06.001. Epub 2020 Jun 10. Mol Ther. 2020. PMID: 32559433 Free PMC article. Clinical Trial.
-
Adeno-associated viral vectors for correction of inborn errors of metabolism: progressing towards clinical application.Curr Pharm Des. 2011;17(24):2500-15. doi: 10.2174/138161211797247569. Curr Pharm Des. 2011. PMID: 21774772 Review.
-
Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy.Hum Gene Ther Methods. 2013 Apr;24(2):59-67. doi: 10.1089/hgtb.2012.243. Epub 2013 Apr 3. Hum Gene Ther Methods. 2013. PMID: 23442094 Free PMC article. Review.
Cited by
-
The seroprevalence of neutralizing antibodies against the adeno-associated virus capsids in Japanese hemophiliacs.Mol Ther Methods Clin Dev. 2022 Oct 27;27:404-414. doi: 10.1016/j.omtm.2022.10.014. eCollection 2022 Dec 8. Mol Ther Methods Clin Dev. 2022. PMID: 36381300 Free PMC article.
-
Immunoadsorption enables successful rAAV5-mediated repeated hepatic gene delivery in nonhuman primates.Blood Adv. 2019 Sep 10;3(17):2632-2641. doi: 10.1182/bloodadvances.2019000380. Blood Adv. 2019. PMID: 31501158 Free PMC article.
-
Method and its Composition for encapsulation, stabilization, and delivery of siRNA in Anionic polymeric nanoplex: An In vitro- In vivo Assessment.Sci Rep. 2019 Nov 5;9(1):16047. doi: 10.1038/s41598-019-52390-4. Sci Rep. 2019. PMID: 31690769 Free PMC article.
-
Advances in gene therapy for hemophilia: basis, current status, and future perspectives.Int J Hematol. 2020 Jan;111(1):31-41. doi: 10.1007/s12185-018-2513-4. Epub 2018 Aug 6. Int J Hematol. 2020. PMID: 30083852 Review.
-
Adeno-Associated Virus Gene Therapy for Hemophilia.Annu Rev Med. 2023 Jan 27;74:231-247. doi: 10.1146/annurev-med-043021-033013. Epub 2022 Sep 14. Annu Rev Med. 2023. PMID: 36103998 Free PMC article. Review.
References
-
- Gaudet D., Méthot J., Déry S., Brisson D., Essiembre C., Tremblay G., Tremblay K., de Wal J., Twisk J., van den Bulk N. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20:361–369. - PMC - PubMed
-
- Grimm D., Zhou S., Nakai H., Thomas C.E., Storm T.A., Fuess S., Matsushita T., Allen J., Surosky R., Lochrie M. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

