Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress
- PMID: 28596115
- PMCID: PMC5555043
- DOI: 10.1016/j.neuroscience.2017.05.049
Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress
Abstract
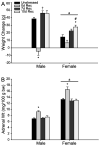
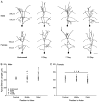
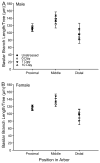
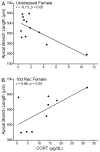
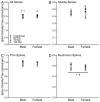
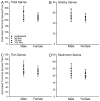
Chronic stress produces differential dendritic remodeling of pyramidal neurons in medial prefrontal cortex of male and female rats. In males, this dendritic remodeling is reversible. However, the timeline of recovery, as well as the potential for reversibility in females, is unknown. Here, we examined dendritic recovery of pyramidal neurons in layer II-II of prelimbic cortex in male and female rats following chronic restraint stress (3h/day for 10days). Dendritic morphology and spine density were analyzed immediately following the cessation of stress, or following a 7- or 10-day recovery period. Chronic stress produced apical dendritic retraction in males, which was coupled with a decrease in the density of stubby spine on apical dendrites. Further, following a 10-day recovery period, the morphology of neurons from stressed rats resembled that of unstressed rats. Male rats given a 7-day recovery period had apical dendritic outgrowth compared to unstressed rats. Immediately after cessation of stress, females showed only minimal dendritic remodeling. The morphology of neurons in stressed females resembled those of unstressed rats following only 7days of recovery, at which time there was also a significant increase in stubby spine density. Males and females also showed different changes in baseline corticosterone concentrations during recovery. These findings not only indicate that dendritic remodeling in prelimbic cortex following chronic stress is different between males and females, but also suggest chronic stress induces differential hypothalamic-pituitary-adrenal axis dysregulation in males and females. These differences may have important implications for responses to subsequent stressors.
Keywords: corticosterone; dendritic morphology; prefrontal cortex; sex differences; spine density.
Copyright © 2017 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery.Neuroscience. 2009 Dec 1;164(2):798-808. doi: 10.1016/j.neuroscience.2009.08.053. Epub 2009 Aug 29. Neuroscience. 2009. PMID: 19723561 Free PMC article.
-
Social instability in adolescence differentially alters dendritic morphology in the medial prefrontal cortex and its response to stress in adult male and female rats.Dev Neurobiol. 2019 Sep;79(9-10):839-856. doi: 10.1002/dneu.22723. Epub 2019 Oct 26. Dev Neurobiol. 2019. PMID: 31612626 Free PMC article.
-
Temporal Dynamics of Acute Stress-Induced Dendritic Remodeling in Medial Prefrontal Cortex and the Protective Effect of Desipramine.Cereb Cortex. 2017 Jan 1;27(1):694-705. doi: 10.1093/cercor/bhv254. Cereb Cortex. 2017. PMID: 26523035
-
Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest.Brain Res. 2009 Oct 13;1293:108-13. doi: 10.1016/j.brainres.2009.03.062. Epub 2009 Apr 8. Brain Res. 2009. PMID: 19361488 Free PMC article. Review.
-
Chronic stress-induced neuroplasticity in the prefrontal cortex: Structural, functional, and molecular mechanisms from development to aging.Brain Res. 2025 Mar 15;1851:149461. doi: 10.1016/j.brainres.2025.149461. Epub 2025 Jan 27. Brain Res. 2025. PMID: 39864644 Review.
Cited by
-
Prefrontal cortical trkB, glucocorticoids, and their interactions in stress and developmental contexts.Neurosci Biobehav Rev. 2018 Dec;95:535-558. doi: 10.1016/j.neubiorev.2018.10.015. Neurosci Biobehav Rev. 2018. PMID: 30477984 Free PMC article. Review.
-
Prelimbic proBDNF Facilitates Retrieval-Dependent Fear Memory Destabilization by Regulation of Synaptic and Neural Functions in Juvenile Rats.Mol Neurobiol. 2022 Jul;59(7):4179-4196. doi: 10.1007/s12035-022-02849-9. Epub 2022 Apr 30. Mol Neurobiol. 2022. PMID: 35501631
-
Post-weaning social isolation causes sex-specific alterations to dendritic spine density in subregions of the prefrontal cortex and nucleus accumbens of adult mice.Brain Res. 2022 Feb 15;1777:147755. doi: 10.1016/j.brainres.2021.147755. Epub 2021 Dec 18. Brain Res. 2022. PMID: 34932973 Free PMC article.
-
Gonadal hormones differentially regulate sex-specific stress effects on glia in the medial prefrontal cortex.J Neuroendocrinol. 2019 Aug;31(8):e12762. doi: 10.1111/jne.12762. Epub 2019 Jul 15. J Neuroendocrinol. 2019. PMID: 31228875 Free PMC article.
-
Pituitary Adenylate Cyclase-Activating Polypeptide in Learning and Memory.Front Cell Neurosci. 2021 Jun 22;15:663418. doi: 10.3389/fncel.2021.663418. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34239418 Free PMC article. Review.
References
-
- Aguilera G, Rabadan-Diehl C, Nikodemova M. Regulation of pituitary corticotropin releasing hormone receptors. Peptides. 2001;22:769–774. - PubMed
-
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830:56–71. - PubMed
-
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: Role of housing conditions. Physiol Behav. 2002;75:661–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

