One-year continuation of copper or levonorgestrel intrauterine devices initiated at the time of emergency contraception
- PMID: 28596121
- PMCID: PMC6040824
- DOI: 10.1016/j.contraception.2017.05.012
One-year continuation of copper or levonorgestrel intrauterine devices initiated at the time of emergency contraception
Abstract
Objective(s): This study compares 1-year intrauterine device (IUD) continuation among women presenting for emergency contraception (EC) and initiating the copper (Cu T380A) IUD or the levonorgestrel (LNG) 52 mg IUD plus 1.5 mg oral LNG.
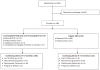
Study design: This cohort study enrolled 188 women who presented at a single family planning clinic in Utah between June 2013 and September 2014 and selected either the Cu T380A IUD or LNG 52 mg IUD plus oral LNG for EC. Trained personnel followed participants by phone, text or e-mail for 12 months or until discontinuation occurred. We assessed reasons for discontinuation and used Cox proportional hazard models, Kaplan-Meier estimates and log-rank tests to assess differences in continuation rates between IUDs.
Results: One hundred seventy-six women received IUDs; 66 (37%) chose the Cu T380A IUD and 110 (63%) chose the LNG 52 mg IUD plus oral LNG. At 1 year, we accounted for 147 (84%) participants, 33 (22%) had requested removals, 13 (9%) had an expulsion and declined reinsertion, 3 (2%) had a pregnancy with their IUD in place and 98 (67%) were still using their device. Continuation rates did not differ by IUD type; 60% of Cu T380A IUD users and 70% of LNG 52 mg IUD plus oral LNG users were still using their device at 12 months (adjusted hazard ratio 0.72, 95% confidence interval 0.40-1.3).
Conclusion(s): Two-thirds of women who chose IUD placement at the EC clinical encounter continued use at 1 year. Women initiating Cu T380A IUD and LNG 52 mg IUD had similar 1-year continuation rates. These findings support same-day insertion of IUDs for women who are seeking EC and would like to use a highly effective reversible method going forward.
Implications: Providing IUD options for EC users presents an opportunity to increase availability of highly effective contraception.
Keywords: Continuation; Copper IUD; Emergency contraception; Levonorgestrel IUD.
Copyright © 2017. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest: D.K.T. receives speaking honoraria from Allergan, Medicines360, Merck and Teva. He serves on advisory boards for Actavis, Bayer, Pharmanest and Teva. The Department of Obstetrics and Gynecology, University of Utah, receives contraceptive clinical trials research funding from Bayer, Bioceptive, Contramed, Medicines360, Merck and Teva. L.M.G. reports personal fees from Evofem Medical Advisory Meeting, outside the submitted work.
Figures


References
-
- Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181–8. - PubMed
-
- Glasier A, Fairhurst K, Wyke S, Ziebland S, Seaman P, Walker J, et al. Advanced provision of emergency contraception does not reduce abortion rates. Contraception. 2004;69:361–6. - PubMed
-
- Polis CB, Schaffer K, Blanchard K, Glasier A, Harper CC, Grimes DA. Advance provision of emergency contraception for pregnancy prevention: a meta-analysis. Obstet Gynecol. 2007;110:1379–88. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

