Disruption of Rab8a and Rab11a causes formation of basolateral microvilli in neonatal enteropathy
- PMID: 28596241
- PMCID: PMC5558269
- DOI: 10.1242/jcs.201897
Disruption of Rab8a and Rab11a causes formation of basolateral microvilli in neonatal enteropathy
Erratum in
-
Correction: Disruption of Rab8a and Rab11a causes formation of basolateral microvilli in neonatal enteropathy (doi: 10.1242/jcs.201897).J Cell Sci. 2018 Sep 17;131(18):jcs224535. doi: 10.1242/jcs.224535. J Cell Sci. 2018. PMID: 30224429 Free PMC article. No abstract available.
Abstract
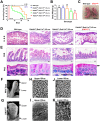
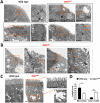
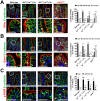
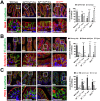
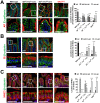
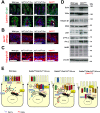
Misplaced formation of microvilli to basolateral domains and intracellular inclusions in enterocytes are pathognomonic features in congenital enteropathy associated with mutation of the apical plasma membrane receptor syntaxin 3 (STX3). Although the demonstrated binding of Myo5b to the Rab8a and Rab11a small GTPases in vitro implicates cytoskeleton-dependent membrane sorting, the mechanisms underlying the microvillar location defect remain unclear. By selective or combinatory disruption of Rab8a and Rab11a membrane traffic in vivo, we demonstrate that transport of distinct cargo to the apical brush border rely on either individual or both Rab regulators, whereas certain basolateral cargos are redundantly transported by both factors. Enterocyte-specific Rab8a and Rab11a double-knockout mouse neonates showed immediate postnatal lethality and more severe enteropathy than single knockouts, with extensive formation of microvilli along basolateral surfaces. Notably, following an inducible Rab11a deletion from neonatal enterocytes, basolateral microvilli were induced within 3 days. These data identify a potentially important and distinct mechanism for a characteristic microvillus defect exhibited by enterocytes of patients with neonatal enteropathy.
Keywords: Enterocyte; Microvillus formation; Neonatal enteropathy; Rab11a; Rab8a.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Aumailley M., Pesch M., Tunggal L., Gaill F. and Fassler R. (2000). Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. J. Cell Sci. 113, 259-268. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

