Pretreatment with indomethacin results in increased heat stroke severity during recovery in a rodent model of heat stroke
- PMID: 28596269
- PMCID: PMC5625077
- DOI: 10.1152/japplphysiol.00242.2017
Pretreatment with indomethacin results in increased heat stroke severity during recovery in a rodent model of heat stroke
Abstract
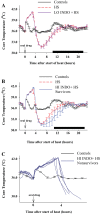
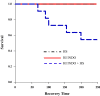
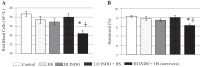
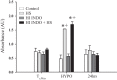
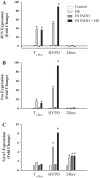
It has been suggested that medications can increase heat stroke (HS) susceptibility/severity. We investigated whether the nonsteroidal anti-inflammatory drug (NSAID) indomethacin (INDO) increases HS severity in a rodent model. Core temperature (Tc) of male, C57BL/6J mice (n = 45) was monitored continuously, and mice were given a dose of INDO [low dose (LO) 1 mg/kg or high dose (HI) 5 mg/kg in flavored treat] or vehicle (flavored treat) before heating. HS animals were heated to 42.4°C and euthanized at three time points for histological, molecular, and metabolic analysis: onset of HS [maximal core temperature (Tc,Max)], 3 h of recovery [minimal core temperature or hypothermia depth (HYPO)], and 24 h of recovery (24 h). Nonheated (control) animals underwent identical treatment in the absence of heat. INDO (LO or HI) had no effect on physiological indicators of performance (e.g., time to Tc,Max, thermal area, or cooling time) during heating or recovery. HI INDO resulted in 45% mortality rate by 24 h (HI INDO + HS group). The gut showed dramatic increases in gross morphological hemorrhage in HI INDO + HS in both survivors and nonsurvivors. HI INDO + HS survivors had significantly lower red blood cell counts and hematocrit suggesting significant hemorrhage. In the liver, HS induced cell death at HYPO and increased inflammation at Tc,Max, HYPO, and 24 h; however, there was additional effect with INDO + HS group. Furthermore, the metabolic profile of the liver was disturbed by heat, but there was no additive effect of INDO + HS. This suggests that there is an increase in morbidity risk with INDO + HS, likely resulting from significant gut injury.NEW & NOTEWORTHY This paper suggests that in a translational mouse model, NSAIDs may be counterindicated in situations that put an individual at risk of heat injury. We show here that a small, single dose of the NSAID indomethacin before heat stroke has a dramatic and highly damaging effect on the gut, which ultimately leads to increased systemic morbidity.
Keywords: NSAID; heat stress; heat stroke; hyperthermia; mouse.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

