Modeling elucidates how refractory period can provide profound nonlinear gain control to graded potential neurons
- PMID: 28596301
- PMCID: PMC5471445
- DOI: 10.14814/phy2.13306
Modeling elucidates how refractory period can provide profound nonlinear gain control to graded potential neurons
Abstract
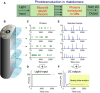
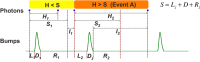
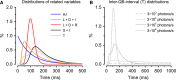
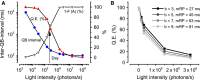
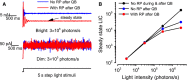
Refractory period (RP) plays a central role in neural signaling. Because it limits an excitable membrane's recovery time from a previous excitation, it can restrict information transmission. Classically, RP means the recovery time from an action potential (spike), and its impact to encoding has been mostly studied in spiking neurons. However, many sensory neurons do not communicate with spikes but convey information by graded potential changes. In these systems, RP can arise as an intrinsic property of their quantal micro/nanodomain sampling events, as recently revealed for quantum bumps (single photon responses) in microvillar photoreceptors. Whilst RP is directly unobservable and hard to measure, masked by the graded macroscopic response that integrates numerous quantal events, modeling can uncover its role in encoding. Here, we investigate computationally how RP can affect encoding of graded neural responses. Simulations in a simple stochastic process model for a fly photoreceptor elucidate how RP can profoundly contribute to nonlinear gain control to achieve a large dynamic range.
Keywords: Drosophila; fly photoreceptor; large dynamic range; neural adaptation; quantal sampling; quantum bump; stochasticity; vision.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures
Similar articles
-
Stochastic, adaptive sampling of information by microvilli in fly photoreceptors.Curr Biol. 2012 Aug 7;22(15):1371-80. doi: 10.1016/j.cub.2012.05.047. Epub 2012 Jun 14. Curr Biol. 2012. PMID: 22704990 Free PMC article.
-
A biomimetic fly photoreceptor model elucidates how stochastic adaptive quantal sampling provides a large dynamic range.J Physiol. 2017 Aug 15;595(16):5439-5456. doi: 10.1113/JP273614. Epub 2017 May 17. J Physiol. 2017. PMID: 28369994 Free PMC article. Review.
-
How a fly photoreceptor samples light information in time.J Physiol. 2017 Aug 15;595(16):5427-5437. doi: 10.1113/JP273645. Epub 2017 Apr 17. J Physiol. 2017. PMID: 28233315 Free PMC article. Review.
-
Latency of phototransduction limits transfer of higher-frequency signals in cockroach photoreceptors.J Neurophysiol. 2020 Jan 1;123(1):120-133. doi: 10.1152/jn.00365.2019. Epub 2019 Nov 13. J Neurophysiol. 2020. PMID: 31721631
-
The rate of information transfer of naturalistic stimulation by graded potentials.J Gen Physiol. 2003 Aug;122(2):191-206. doi: 10.1085/jgp.200308824. Epub 2003 Jul 14. J Gen Physiol. 2003. PMID: 12860926 Free PMC article.
References
-
- Blomfield, S. 1974. Arithmetical operations performed by nerve‐cells. Brain Res. 69:115–124. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

