Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474
- PMID: 28596366
- PMCID: PMC5641481
- DOI: 10.1126/science.aaf7497
Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474
Abstract
A recent phase 1 trial of the fatty acid amide hydrolase (FAAH) inhibitor BIA 10-2474 led to the death of one volunteer and produced mild-to-severe neurological symptoms in four others. Although the cause of the clinical neurotoxicity is unknown, it has been postulated, given the clinical safety profile of other tested FAAH inhibitors, that off-target activities of BIA 10-2474 may have played a role. Here we use activity-based proteomic methods to determine the protein interaction landscape of BIA 10-2474 in human cells and tissues. This analysis revealed that the drug inhibits several lipases that are not targeted by PF04457845, a highly selective and clinically tested FAAH inhibitor. BIA 10-2474, but not PF04457845, produced substantial alterations in lipid networks in human cortical neurons, suggesting that promiscuous lipase inhibitors have the potential to cause metabolic dysregulation in the nervous system.
Copyright © 2017, American Association for the Advancement of Science.
Figures
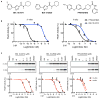
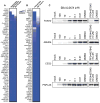

Comment in
-
Enzymology: Tracking off-targets.Nat Chem Biol. 2017 Jul 18;13(8):817. doi: 10.1038/nchembio.2445. Nat Chem Biol. 2017. PMID: 28853735 No abstract available.
References
-
- Butler D, Callaway E. Scientists in the dark after French clinical trial proves fatal. Nature. 2016;529:263–264. - PubMed
-
- Kerbrat A, et al. Acute Neurologic Disorder from an Inhibitor of Fatty Acid Amide Hydrolase. N Engl J Med. 2016;375:1717–1725. - PubMed
-
- Bégaud B, et al. Report by the Temporary Specialist Scientific Committee (TSSC), “FAAH (Fatty Acid Amide Hydrolase)”, on the causes of the accident during a Phase 1 clinical trial. 2016:1–28.
-
- Cravatt BF, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

