Leukocyte Bim deficiency does not impact atherogenesis in ldlr -/- mice, despite a pronounced induction of autoimmune inflammation
- PMID: 28596542
- PMCID: PMC5465223
- DOI: 10.1038/s41598-017-02771-4
Leukocyte Bim deficiency does not impact atherogenesis in ldlr -/- mice, despite a pronounced induction of autoimmune inflammation
Abstract
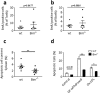
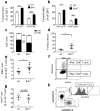
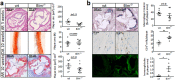
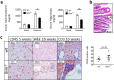
Proapoptotic Bcl-2 family member Bim is particularly relevant for deletion of autoreactive and activated T and B cells, implicating Bim in autoimmunity. As atherosclerosis is a chronic inflammatory process with features of autoimmune disease, we investigated the impact of hematopoietic Bim deficiency on plaque formation and parameters of plaque stability. Bim -/- or wild type bone marrow transplanted ldlr -/- mice were fed a Western type diet (WTD) for 5 or 10 weeks, after which they were immunophenotyped and atherosclerotic lesions were analyzed. Bim -/- transplanted mice displayed splenomegaly and overt lymphocytosis. CD4+ and CD8+ T cells were more activated (increased CD69 and CD71 expression, increased interferon gamma production). B cells were elevated by 147%, with a shift towards the pro-atherogenic IgG-producing B2 cell phenotype, resulting in a doubling of anti-oxLDL IgG1 antibody titers in serum of bim -/- mice. Bim -/- mice displayed massive intraplaque accumulation of Ig complexes and of lesional T cells, although this did not translate in changes in plaque size or stability features (apoptotic cell and macrophage content). The surprising lack in plaque phenotype despite the profound pro-atherogenic immune effects may be attributable to the sharp reduction of serum cholesterol levels in WTD fed bim -/- mice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Apolipoprotein A1 deficiency increases macrophage apoptosis and necrotic core development in atherosclerotic plaques in a Bim-dependent manner.J Lipid Res. 2025 May;66(5):100782. doi: 10.1016/j.jlr.2025.100782. Epub 2025 Mar 20. J Lipid Res. 2025. PMID: 40120762 Free PMC article.
-
Hematopoietic arginase 1 deficiency results in decreased leukocytosis and increased foam cell formation but does not affect atherosclerosis.Atherosclerosis. 2017 Jan;256:35-46. doi: 10.1016/j.atherosclerosis.2016.11.018. Epub 2016 Nov 16. Atherosclerosis. 2017. PMID: 27998825
-
Hematopoietic α7 nicotinic acetylcholine receptor deficiency increases inflammation and platelet activation status, but does not aggravate atherosclerosis.J Thromb Haemost. 2015 Jan;13(1):126-35. doi: 10.1111/jth.12765. Epub 2014 Nov 13. J Thromb Haemost. 2015. PMID: 25345495
-
Lack of myeloid Fatp1 increases atherosclerotic lesion size in Ldlr-/- mice.Atherosclerosis. 2017 Nov;266:182-189. doi: 10.1016/j.atherosclerosis.2017.10.009. Epub 2017 Oct 7. Atherosclerosis. 2017. PMID: 29035781 Free PMC article.
-
Orp8 deficiency in bone marrow-derived cells reduces atherosclerotic lesion progression in LDL receptor knockout mice.PLoS One. 2014 Oct 27;9(10):e109024. doi: 10.1371/journal.pone.0109024. eCollection 2014. PLoS One. 2014. PMID: 25347070 Free PMC article.
Cited by
-
Apolipoprotein A1 deficiency increases macrophage apoptosis and necrotic core development in atherosclerotic plaques in a Bim-dependent manner.J Lipid Res. 2025 May;66(5):100782. doi: 10.1016/j.jlr.2025.100782. Epub 2025 Mar 20. J Lipid Res. 2025. PMID: 40120762 Free PMC article.
-
Gene Expression Profiling of Apoptotic Proteins in Circulating Peripheral Blood Mononuclear Cells in Type II Diabetes Mellitus and Modulation by Metformin.Diabetes Metab Syndr Obes. 2021 Mar 15;14:1129-1139. doi: 10.2147/DMSO.S300048. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 33758522 Free PMC article.
-
The role of immunoglobins in atherosclerosis development; friends or foe?Mol Cell Biochem. 2025 May;480(5):2737-2747. doi: 10.1007/s11010-024-05158-y. Epub 2024 Nov 27. Mol Cell Biochem. 2025. PMID: 39592554 Review.
-
Low human and murine Mcl-1 expression leads to a pro-apoptotic plaque phenotype enriched in giant-cells.Sci Rep. 2019 Oct 10;9(1):14547. doi: 10.1038/s41598-019-51020-3. Sci Rep. 2019. PMID: 31601924 Free PMC article.
-
Apoptotic cell death in disease-Current understanding of the NCCD 2023.Cell Death Differ. 2023 May;30(5):1097-1154. doi: 10.1038/s41418-023-01153-w. Epub 2023 Apr 26. Cell Death Differ. 2023. PMID: 37100955 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

