Sortase A-mediated crosslinked short-chain dehydrogenases/reductases as novel biocatalysts with improved thermostability and catalytic efficiency
- PMID: 28596548
- PMCID: PMC5465079
- DOI: 10.1038/s41598-017-03168-z
Sortase A-mediated crosslinked short-chain dehydrogenases/reductases as novel biocatalysts with improved thermostability and catalytic efficiency
Abstract
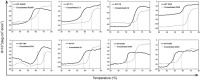
(S)-carbonyl reductase II (SCRII) from Candida parapsilosis is a short-chain alcohol dehydrogenase/reductase. It catalyses the conversion of 2-hydroxyacetophenone to (S)-1-phenyl-1,2-ethanediol with low efficiency. Sortase was reported as a molecular "stapler" for site-specific protein conjugation to strengthen or add protein functionality. Here, we describe Staphylococcus aureus sortase A-mediated crosslinking of SCRII to produce stable catalysts for efficient biotransformation. Via a native N-terminal glycine and an added GGGGSLPETGG peptide at C-terminus of SCRII, SCRII subunits were conjugated by sortase A to form crosslinked SCRII, mainly dimers and trimers. The crosslinked SCRII showed over 6-fold and 4-fold increases, respectively, in activity and k cat/K m values toward 2-hydroxyacetophenone compared with wild-type SCRII. Moreover, crosslinked SCRII was much more thermostable with its denaturation temperature (Tm) increased to 60 °C. Biotransformation result showed that crosslinked SCRII gave a product optical purity of 100% and a yield of >99.9% within 3 h, a 16-fold decrease in transformation duration with respect to Escherichia coli/pET-SCRII. Sortase A-catalysed ligation also obviously improved Tms and product yields of eight other short-chain alcohol dehydrogenases/reductases. This work demonstrates a generic technology to improve enzyme function and thermostability through sortase A-mediated crosslinking of oxidoreductases.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Kara S, et al. Access to Lactone Building Blocks via Horse Liver Alcohol Dehydrogenase-Catalyzed Oxidative Lactonization. ACS Catal. 2013;3:2436–2439. doi: 10.1021/cs400535c. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

