Transgenic overexpression of GTP cyclohydrolase 1 in cardiomyocytes ameliorates post-infarction cardiac remodeling
- PMID: 28596578
- PMCID: PMC5465102
- DOI: 10.1038/s41598-017-03234-6
Transgenic overexpression of GTP cyclohydrolase 1 in cardiomyocytes ameliorates post-infarction cardiac remodeling
Abstract
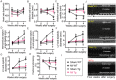
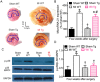
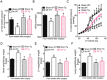
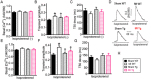
GTP cyclohydrolase 1 (GCH1) and its product tetrahydrobiopterin play crucial roles in cardiovascular health and disease, yet the exact regulation and role of GCH1 in adverse cardiac remodeling after myocardial infarction are still enigmatic. Here we report that cardiac GCH1 is degraded in remodeled hearts after myocardial infarction, concomitant with increases in the thickness of interventricular septum, interstitial fibrosis, and phosphorylated p38 mitogen-activated protein kinase and decreases in left ventricular anterior wall thickness, cardiac contractility, tetrahydrobiopterin, the dimers of nitric oxide synthase, sarcoplasmic reticulum Ca2+ release, and the expression of sarcoplasmic reticulum Ca2+ handling proteins. Intriguingly, transgenic overexpression of GCH1 in cardiomyocytes reduces the thickness of interventricular septum and interstitial fibrosis and increases anterior wall thickness and cardiac contractility after infarction. Moreover, we show that GCH1 overexpression decreases phosphorylated p38 mitogen-activated protein kinase and elevates tetrahydrobiopterin levels, the dimerization and phosphorylation of neuronal nitric oxide synthase, sarcoplasmic reticulum Ca2+ release, and sarcoplasmic reticulum Ca2+ handling proteins in post-infarction remodeled hearts. Our results indicate that the pivotal role of GCH1 overexpression in post-infarction cardiac remodeling is attributable to preservation of neuronal nitric oxide synthase and sarcoplasmic reticulum Ca2+ handling proteins, and identify a new therapeutic target for cardiac remodeling after infarction.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Townsend N, et al. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;0:14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

