Tetraploid embryonic stem cells can contribute to the development of chimeric fetuses and chimeric extraembryonic tissues
- PMID: 28596585
- PMCID: PMC5465063
- DOI: 10.1038/s41598-017-02783-0
Tetraploid embryonic stem cells can contribute to the development of chimeric fetuses and chimeric extraembryonic tissues
Abstract
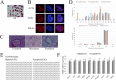
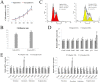
Our study examined the in vivo chimeric and survival capacities of chimeras created by injecting tetraploid embryonic stem cells (ESCs) expressing green fluorescent protein (GFP) into diploid embryos. At 3.5 days post-coitum (dpc) and 4.5 dpc, the tetraploid ESCs were able to contribute to the inner cell mass (ICM) just as diploid ESCs tagged with GFP. At 6.5 dpc, 8.0 dpc and 10.5 dpc, the tetraploid ESCs manifested in the same location as the diploid ESCs. The GFP cells in the extraembryonic tissues and fetuses of tetraploid ESC chimeras were tetraploid as determined by fluorescence activated cell sorting (FACS). Furthermore, tetraploid ESCs contributed to the development of the placenta, embryolemma and umbilical cord at 13.5 dpc and 16.5 dpc; however, very less GFP cells were found in the fetuses of tetraploid ESC chimeras. We further found that the proliferation of tetraploid ESCs was slower than that of diploid ESCs. In addition, the relative mRNA expression in the three germ layers and the trophoblast was abnormal in the EBs of tetraploid ESCs compared with diploid ESCs. In short, slower proliferation and abnormal differentiation potential of tetraploid ESCs might be two of the reasons for their poor survival and chimeric capacities.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Generating chimeric mice from embryonic stem cells via vial coculturing or hypertonic microinjection.Methods Mol Biol. 2014;1194:77-111. doi: 10.1007/978-1-4939-1215-5_5. Methods Mol Biol. 2014. PMID: 25064099
-
Tetraploid Embryonic Stem Cells Maintain Pluripotency and Differentiation Potency into Three Germ Layers.PLoS One. 2015 Jun 19;10(6):e0130585. doi: 10.1371/journal.pone.0130585. eCollection 2015. PLoS One. 2015. PMID: 26091100 Free PMC article.
-
Development of rat tetraploid and chimeric embryos aggregated with diploid cells.Zygote. 2006 Nov;14(4):287-97. doi: 10.1017/S096719940600387X. Zygote. 2006. PMID: 17266787
-
Genetic control of extraembryonic cell lineages studied with tetraploid<-->diploid chimeric concepti.Biochem Cell Biol. 1998;76(6):1017-27. Biochem Cell Biol. 1998. PMID: 10392713 Review.
-
Uncovering the true identity of naïve pluripotent stem cells.Trends Cell Biol. 2013 Sep;23(9):442-8. doi: 10.1016/j.tcb.2013.04.004. Epub 2013 May 17. Trends Cell Biol. 2013. PMID: 23685019 Review.
Cited by
-
Paraffin-embedded vertical sections of mouse embryonic stem cells.J Vet Med Sci. 2018 Oct 10;80(10):1479-1481. doi: 10.1292/jvms.18-0352. Epub 2018 Aug 9. J Vet Med Sci. 2018. PMID: 30089742 Free PMC article.
-
A Survey of Essential Genome Stability Genes Reveals That Replication Stress Mitigation Is Critical for Peri-Implantation Embryogenesis.Front Cell Dev Biol. 2020 May 29;8:416. doi: 10.3389/fcell.2020.00416. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32548123 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

