AAV-Mediated Gene Supplementation Therapy in Achromatopsia Type 2: Preclinical Data on Therapeutic Time Window and Long-Term Effects
- PMID: 28596720
- PMCID: PMC5442229
- DOI: 10.3389/fnins.2017.00292
AAV-Mediated Gene Supplementation Therapy in Achromatopsia Type 2: Preclinical Data on Therapeutic Time Window and Long-Term Effects
Abstract
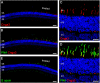
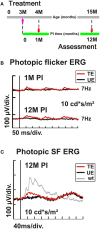
Achromatopsia type 2 (ACHM2) is a severe, inherited eye disease caused by mutations in the CNGA3 gene encoding the α subunit of the cone photoreceptor cyclic nucleotide-gated (CNG) channel. Patients suffer from strongly impaired daylight vision, photophobia, nystagmus, and lack of color discrimination. We have previously shown in the Cnga3 knockout (KO) mouse model of ACHM2 that gene supplementation therapy is effective in rescuing cone function and morphology and delaying cone degeneration. In our preclinical approach, we use recombinant adeno-associated virus (AAV) vector-mediated gene transfer to express the murine Cnga3 gene under control of the mouse blue opsin promoter. Here, we provide novel data on the efficiency and permanence of such gene supplementation therapy in Cnga3 KO mice. Specifically, we compare the influence of two different AAV vector capsids, AAV2/5 (Y719F) and AAV2/8 (Y733F), on restoration of cone function, and assess the effect of age at time of treatment on the long-term outcome. The evaluation included in vivo analysis of retinal function using electroretinography (ERG) and immunohistochemical analysis of vector-driven Cnga3 transgene expression. We found that both vector capsid serotypes led to a comparable rescue of cone function over the observation period between 4 weeks and 3 months post treatment. In addition, a clear therapeutic effect was present in mice treated at 2 weeks of age as well as in mice treated at 3 months of age at the first assessment at 4 weeks after treatment. Importantly, the effect extended in both cases over the entire observation period of 12 months post treatment. However, the average ERG amplitude levels differed between the two groups, suggesting a role of the absolute age, or possibly, the associated state of the degeneration, on the achievable outcome. In summary, we found that the therapeutic time window of opportunity for AAV-mediated Cnga3 gene supplementation therapy in the Cnga3 KO mouse model extends at least to an age of 3 months, but is presumably limited by the condition, number and topographical distribution of remaining cones at the time of treatment. No impact of the choice of capsid on the therapeutic success was detected.
Keywords: AAV vector; CNGA3; achromatopsia; cone function restoration; gene therapy; non-invasive diagnostic techniques; photoreceptor cells; subretinal delivery.
Figures




Similar articles
-
Long-term retinal cone rescue using a capsid mutant AAV8 vector in a mouse model of CNGA3-achromatopsia.PLoS One. 2017 Nov 13;12(11):e0188032. doi: 10.1371/journal.pone.0188032. eCollection 2017. PLoS One. 2017. PMID: 29131863 Free PMC article.
-
Vitreal delivery of AAV vectored Cnga3 restores cone function in CNGA3-/-/Nrl-/- mice, an all-cone model of CNGA3 achromatopsia.Hum Mol Genet. 2015 Jul 1;24(13):3699-707. doi: 10.1093/hmg/ddv114. Epub 2015 Apr 8. Hum Mol Genet. 2015. PMID: 25855802 Free PMC article.
-
Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial.JAMA Ophthalmol. 2020 Jun 1;138(6):643-651. doi: 10.1001/jamaophthalmol.2020.1032. JAMA Ophthalmol. 2020. PMID: 32352493 Free PMC article. Clinical Trial.
-
[Gene replacement therapy in achromatopsia type 2].Klin Monbl Augenheilkd. 2014 Mar;231(3):232-40. doi: 10.1055/s-0034-1368180. Epub 2014 Mar 21. Klin Monbl Augenheilkd. 2014. PMID: 24658860 Review. German.
-
Gene therapy for achromatopsia.J Gene Med. 2017 Mar;19(3). doi: 10.1002/jgm.2944. J Gene Med. 2017. PMID: 28095637 Review.
Cited by
-
Oscillatory Potentials in Achromatopsia as a Tool for Understanding Cone Retinal Functions.Int J Mol Sci. 2021 Nov 24;22(23):12717. doi: 10.3390/ijms222312717. Int J Mol Sci. 2021. PMID: 34884517 Free PMC article.
-
Gene Therapy in Inherited Retinal Diseases: An Update on Current State of the Art.Front Med (Lausanne). 2021 Oct 15;8:750586. doi: 10.3389/fmed.2021.750586. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34722588 Free PMC article. Review.
-
Achromatopsia-Visual Cortex Stability and Plasticity in the Absence of Functional Cones.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):23. doi: 10.1167/iovs.64.13.23. Invest Ophthalmol Vis Sci. 2023. PMID: 37847226 Free PMC article.
-
Clinical applications of retinal gene therapies.Precis Clin Med. 2018 Jun;1(1):5-20. doi: 10.1093/pcmedi/pby004. Epub 2018 Jun 1. Precis Clin Med. 2018. PMID: 35694125 Free PMC article. Review.
-
Biology, Pathobiology and Gene Therapy of CNG Channel-Related Retinopathies.Biomedicines. 2023 Jan 19;11(2):269. doi: 10.3390/biomedicines11020269. Biomedicines. 2023. PMID: 36830806 Free PMC article. Review.
References
-
- Aligianis I. A., Forshew T., Johnson S., Michaelides M., Johnson C. A., Trembath R. C., et al. . (2002). Mapping of a novel locus for achromatopsia (ACHM4) to 1p and identification of a germline mutation in the alpha subunit of cone transducin (GNAT2). J. Med. Genet. 39, 656–660. 10.1136/jmg.39.9.656 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

