RANK/RANKL/OPG Signalization Implication in Periodontitis: New Evidence from a RANK Transgenic Mouse Model
- PMID: 28596739
- PMCID: PMC5442248
- DOI: 10.3389/fphys.2017.00338
RANK/RANKL/OPG Signalization Implication in Periodontitis: New Evidence from a RANK Transgenic Mouse Model
Abstract
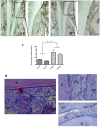
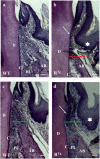
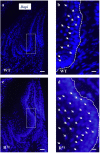
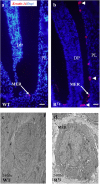
Periodontitis is based on a complex inflammatory over-response combined with possible genetic predisposition factors. The RANKL/RANK/OPG signaling pathway is implicated in bone resorption through its key function in osteoclast differentiation and activation, as well as in the inflammatory response. This central element of osteo-immunology has been suggested to be perturbed in several diseases, including periodontitis, as it is a predisposing factor for this disease. The aim of the present study was to validate this hypothesis using a transgenic mouse line, which over-expresses RANK (RTg) and develops a periodontitis-like phenotype at 5 months of age. RTg mice exhibited severe alveolar bone loss, an increased number of TRAP positive cells, and disorganization of periodontal ligaments. This phenotype was more pronounced in females. We also observed dental root resorption lacunas. Hyperplasia of the gingival epithelium, including Malassez epithelial rests, was visible as early as 25 days, preceding any other symptoms. These results demonstrate that perturbations of the RANKL/RANK/OPG system constitute a core element of periodontitis, and more globally, osteo-immune diseases.
Keywords: RANK; alveolar bone; gingival epithelium; malassez epithelial rests (MER); osteoclasts; periodontitis; root resorption.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

