The Retina in Multiple System Atrophy: Systematic Review and Meta-Analysis
- PMID: 28596752
- PMCID: PMC5443142
- DOI: 10.3389/fneur.2017.00206
The Retina in Multiple System Atrophy: Systematic Review and Meta-Analysis
Abstract
Background: Multiple system atrophy (MSA) is a rare, adult-onset, rapidly progressive fatal synucleinopathy that primarily affects oligodendroglial cells in the brain. Patients with MSA only rarely have visual complaints, but recent studies of the retina using optical coherence tomography (OCT) showed atrophy of the peripapillary retinal nerve fiber layer (RNFL) and to a lesser extent the macular ganglion cell layer (GCL) complex.
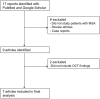
Methods: We performed a literature review and meta-analysis according to the preferred reporting items for systematic reviews and meta-analyses guidelines for studies published before January 2017, identified through PubMed and Google Scholar databases, which reported OCT-related outcomes in patients with MSA and controls. A random-effects model was constructed.
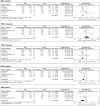
Results: The meta-analysis search strategy yielded 15 articles of which 7 met the inclusion criteria. The pooled difference in the average thickness of the RNFL was -5.48 μm (95% CI, -6.23 to -4.73; p < 0.0001), indicating significant thinning in patients with MSA. The pooled results showed significant thinning in all the specific RNFL quadrants, except in the temporal RNFL quadrant, where the thickness in MSA and controls was similar [pooled difference of 1.11 µm (95% CI, -4.03 to 6.26; p = 0.67)]. This pattern of retinal damage suggests that MSA patients have preferential loss of retinal ganglion cells projecting to the magnocellular pathway (M-cells), which are mainly located in the peripheral retina and are not essential for visual acuity. Visual acuity, on the other hand, relies mostly on macular ganglion cells projecting to the parvocellular pathway (P-cells) through the temporal portion of the RNFL, which are relatively spared in MSA patients.
Conclusion: The retinal damage in patients with MSA differs from that observed in patients with Parkinson disease (PD). Patients with MSA have more relative preservation of temporal sector of the RNFL and less severe atrophy of the macular GCL complex. We hypothesize that in patients with MSA there is predominant damage of large myelinated optic nerve axons like those originating from the M-cells. These large axons may require higher support from oligodendrocytes. Conversely, in patients with PD, P-cells might be more affected.
Keywords: alpha-synuclein; ganglion cell layer; multiple system atrophy; optical coherence tomography; retina; retinal nerve fiber layer.
Figures



Similar articles
-
Retinal thinning correlates with clinical severity in multiple system atrophy.J Neurol. 2016 Oct;263(10):2039-47. doi: 10.1007/s00415-016-8230-0. Epub 2016 Jul 14. J Neurol. 2016. PMID: 27416856
-
[Progression of nerve fiber layer defects in retrobulbar optic neuritis by the macular ganglion cell complex].J Fr Ophtalmol. 2017 Nov;40(9):777-787. doi: 10.1016/j.jfo.2017.06.003. Epub 2017 Oct 16. J Fr Ophtalmol. 2017. PMID: 29050924 French.
-
Progressive retinal structure abnormalities in multiple system atrophy.Mov Disord. 2015 Dec;30(14):1944-53. doi: 10.1002/mds.26360. Epub 2015 Sep 11. Mov Disord. 2015. PMID: 26359930 Free PMC article.
-
Optical Coherence Tomography in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis.Front Neurol. 2020 Oct 16;11:578698. doi: 10.3389/fneur.2020.578698. eCollection 2020. Front Neurol. 2020. PMID: 33178120 Free PMC article.
-
Evaluation of retina and microvascular changes in the patient with Parkinson's disease: A systematic review and meta-analysis.Front Med (Lausanne). 2022 Sep 15;9:957700. doi: 10.3389/fmed.2022.957700. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36186761 Free PMC article.
Cited by
-
[Retinal OCT biomarkers and neurodegenerative diseases of the central nervous system beyond Alzheimer's disease].Ophthalmologie. 2024 Feb;121(2):93-104. doi: 10.1007/s00347-023-01974-7. Epub 2024 Jan 23. Ophthalmologie. 2024. PMID: 38263475 Review. German.
-
Diagnosis of multiple sclerosis using multifocal ERG data feature fusion.Inf Fusion. 2021 Dec;76:157-167. doi: 10.1016/j.inffus.2021.05.006. Inf Fusion. 2021. PMID: 34867127 Free PMC article.
-
Optical Coherence Tomography Angiography in Neurodegenerative Diseases: A Review.Eye Brain. 2020 Jul 14;12:73-87. doi: 10.2147/EB.S193026. eCollection 2020. Eye Brain. 2020. PMID: 32765149 Free PMC article. Review.
-
Retinal Structure Abnormalities in Parkinson's Disease and Atypical Parkinsonism.Biomolecules. 2023 Jan 23;13(2):218. doi: 10.3390/biom13020218. Biomolecules. 2023. PMID: 36830588 Free PMC article.
-
α-Synuclein pathology in post-mortem retina and optic nerve is specific for α-synucleinopathies.NPJ Parkinsons Dis. 2023 Aug 28;9(1):124. doi: 10.1038/s41531-023-00570-5. NPJ Parkinsons Dis. 2023. PMID: 37640753 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

