Enzymatic Degradation of Aromatic and Aliphatic Polyesters by P. pastoris Expressed Cutinase 1 from Thermobifida cellulosilytica
- PMID: 28596765
- PMCID: PMC5443175
- DOI: 10.3389/fmicb.2017.00938
Enzymatic Degradation of Aromatic and Aliphatic Polyesters by P. pastoris Expressed Cutinase 1 from Thermobifida cellulosilytica
Abstract
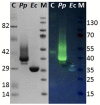
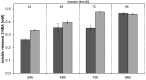
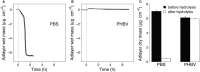
To study hydrolysis of aromatic and aliphatic polyesters cutinase 1 from Thermobifida cellulosilytica (Thc_Cut1) was expressed in P. pastoris. No significant differences between the expression of native Thc_Cut1 and of two glycosylation site knock out mutants (Thc_Cut1_koAsn and Thc_Cut1_koST) concerning the total extracellular protein concentration and volumetric activity were observed. Hydrolysis of poly(ethylene terephthalate) (PET) was shown for all three enzymes based on quantification of released products by HPLC and similar concentrations of released terephthalic acid (TPA) and mono(2-hydroxyethyl) terephthalate (MHET) were detected for all enzymes. Both tested aliphatic polyesters poly(butylene succinate) (PBS) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) were hydrolyzed by Thc_Cut1 and Thc_Cut1_koST, although PBS was hydrolyzed to significantly higher extent than PHBV. These findings were also confirmed via quartz crystal microbalance (QCM) analysis; for PHBV only a small mass change was observed while the mass of PBS thin films decreased by 93% upon enzymatic hydrolysis with Thc_Cut1. Although both enzymes led to similar concentrations of released products upon hydrolysis of PET and PHBV, Thc_Cut1_koST was found to be significantly more active on PBS than the native Thc_Cut1. Hydrolysis of PBS films by Thc_Cut1 and Thc_Cut1_koST was followed by weight loss and scanning electron microscopy (SEM). Within 96 h of hydrolysis up to 92 and 41% of weight loss were detected with Thc_Cut1_koST and Thc_Cut1, respectively. Furthermore, SEM characterization of PBS films clearly showed that enzyme tretment resulted in morphological changes of the film surface.
Keywords: Pichia pastoris; Thermobifida cellulosilytica; aliphatic polyesters; cutinase; enzymatic hydrolysis; poly(3-hydroxybutyrate-co-3-hydroxyvalerate); poly(butylene succinate); poly(ethylene terephthalate).
Figures







References
-
- Bretthauer R. K., Castellino F. J. (1999). Glycosylation of Pichia pastoris-derived proteins. Biotechnol. Appl. Biochem. 30(Pt 3), 193–200. - PubMed
-
- Brueckner T., Eberl A., Heumann S., Rabe M., Guebitz M. G. (2008). Enzymatic and chemical hydrolysis of poly(ethylene terephthalate) fabrics. Polym. Chem. 46, 6435–6443. 10.1002/pola.22952 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

