A Phase 1 Human Immunodeficiency Virus Vaccine Trial for Cross-Profiling the Kinetics of Serum and Mucosal Antibody Responses to CN54gp140 Modulated by Two Homologous Prime-Boost Vaccine Regimens
- PMID: 28596770
- PMCID: PMC5442169
- DOI: 10.3389/fimmu.2017.00595
A Phase 1 Human Immunodeficiency Virus Vaccine Trial for Cross-Profiling the Kinetics of Serum and Mucosal Antibody Responses to CN54gp140 Modulated by Two Homologous Prime-Boost Vaccine Regimens
Abstract
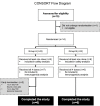
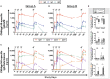
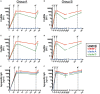
A key aspect to finding an efficacious human immunodeficiency virus (HIV) vaccine is the optimization of vaccine schedules that can mediate the efficient maturation of protective immune responses. In the present study, we investigated the effect of alternate booster regimens on the immune responses to a candidate HIV-1 clade C CN54gp140 envelope protein, which was coadministered with the TLR4-agonist glucopyranosyl lipid A-aqueous formulation. Twelve study participants received a common three-dose intramuscular priming series followed by a final booster at either 6 or 12 months. The two homologous prime-boost regimens were well tolerated and induced CN54gp140-specific responses that were observed in both the systemic and mucosal compartments. Levels of vaccine-induced IgG-subclass antibodies correlated significantly with FcγR engagement, and both vaccine regimens were associated with strikingly similar patterns in antibody titer and FcγR-binding profiles. In both groups, identical changes in the antigen (Ag)-specific IgG-subclass fingerprint, leading to a decrease in IgG1 and an increase in IgG4 levels, were modulated by booster injections. Here, the dissection of immune profiles further supports the notion that prime-boost strategies are essential for the induction of diverse Ag-specific HIV-1 responses. The results reported here clearly demonstrate that identical responses were effectively and safely induced by both vaccine regimens, indicating that an accelerated 6-month regimen could be employed for the rapid induction of immune responses against CN54gp140 with no apparent impact on the overall quality of the induced immune response. (This study has been registered at http://ClinicalTrials.gov under registration no. NCT01966900.).
Keywords: IgG subclasses; adjuvant; homologous prime-boost strategy; human immunodeficiency virus envelope protein; human immunodeficiency virus vaccine; mucosal compartment; vaccine interval.
Figures



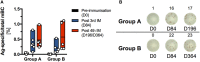
Similar articles
-
Optimized Mucosal Modified Vaccinia Virus Ankara Prime/Soluble gp120 Boost HIV Vaccination Regimen Induces Antibody Responses Similar to Those of an Intramuscular Regimen.J Virol. 2019 Jun 28;93(14):e00475-19. doi: 10.1128/JVI.00475-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31068425 Free PMC article.
-
Priming with a Potent HIV-1 DNA Vaccine Frames the Quality of Immune Responses prior to a Poxvirus and Protein Boost.J Virol. 2019 Jan 17;93(3):e01529-18. doi: 10.1128/JVI.01529-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429343 Free PMC article.
-
Heterologous prime-boost regimens with a recombinant chimpanzee adenoviral vector and adjuvanted F4 protein elicit polyfunctional HIV-1-specific T-Cell responses in macaques.PLoS One. 2015 Apr 9;10(4):e0122835. doi: 10.1371/journal.pone.0122835. eCollection 2015. PLoS One. 2015. PMID: 25856308 Free PMC article.
-
Potential To Streamline Heterologous DNA Prime and NYVAC/Protein Boost HIV Vaccine Regimens in Rhesus Macaques by Employing Improved Antigens.J Virol. 2016 Mar 28;90(8):4133-4149. doi: 10.1128/JVI.03135-15. Print 2016 Apr. J Virol. 2016. PMID: 26865719 Free PMC article.
-
New prospects for the development of a vaccine against human immunodeficiency virus type 1. An overview.C R Acad Sci III. 1999 Nov;322(11):959-66. doi: 10.1016/s0764-4469(00)87193-0. C R Acad Sci III. 1999. PMID: 10646090 Review.
Cited by
-
Envelope-Specific Recognition Patterns of HIV Vaccine-Induced IgG Antibodies Are Linked to Immunogen Structure and Sequence.Front Immunol. 2019 Apr 24;10:717. doi: 10.3389/fimmu.2019.00717. eCollection 2019. Front Immunol. 2019. PMID: 31105688 Free PMC article.
-
Immunoglobulin G1 Allotype Influences Antibody Subclass Distribution in Response to HIV gp140 Vaccination.Front Immunol. 2017 Dec 20;8:1883. doi: 10.3389/fimmu.2017.01883. eCollection 2017. Front Immunol. 2017. PMID: 29326728 Free PMC article.
-
A systems approach to elucidate personalized mechanistic complexities of antibody-Fc receptor activation post-vaccination.Cell Rep Med. 2021 Sep 1;2(9):100386. doi: 10.1016/j.xcrm.2021.100386. eCollection 2021 Sep 21. Cell Rep Med. 2021. PMID: 34622227 Free PMC article. Clinical Trial.
-
A Quantitative Approach to Unravel the Role of Host Genetics in IgG-FcγR Complex Formation After Vaccination.Front Immunol. 2022 Feb 22;13:820148. doi: 10.3389/fimmu.2022.820148. eCollection 2022. Front Immunol. 2022. PMID: 35273603 Free PMC article.
-
Induction of Identical IgG HIV-1 Envelope Epitope Recognition Patterns After Initial HIVIS-DNA/MVA-CMDR Immunization and a Late MVA-CMDR Boost.Front Immunol. 2020 Apr 28;11:719. doi: 10.3389/fimmu.2020.00719. eCollection 2020. Front Immunol. 2020. PMID: 32411138 Free PMC article. Clinical Trial.
References
-
- UNAIDS. Fact Sheet - Latest Statistics on the Status of the Aids Epidemic [Online]. UNAIDS (2016). Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_e... (accessed May 11, 2017).
-
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis (2006) 194:1661–71.10.1086/508748 - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

