Katanin Effects on Dynamics of Cortical Microtubules and Mitotic Arrays in Arabidopsis thaliana Revealed by Advanced Live-Cell Imaging
- PMID: 28596780
- PMCID: PMC5443160
- DOI: 10.3389/fpls.2017.00866
Katanin Effects on Dynamics of Cortical Microtubules and Mitotic Arrays in Arabidopsis thaliana Revealed by Advanced Live-Cell Imaging
Abstract
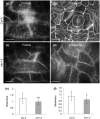
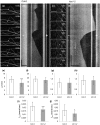
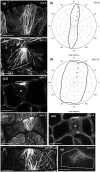
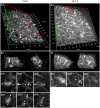
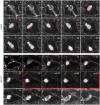
Katanin is the only microtubule severing protein identified in plants so far. Previous studies have documented its role in regulating cortical microtubule organization during cell growth and morphogenesis. Although, some cell division defects are reported in KATANIN mutants, it is not clear whether or how katanin activity may affect microtubule dynamics in interphase cells, as well as the progression of mitosis and cytokinesis and the orientation of cell division plane (CDP). For this reason, we characterized microtubule organization and dynamics in growing and dividing cotyledon cells of Arabidopsis ktn1-2 mutant devoid of KATANIN 1 activity. In interphase epidermal cells of ktn1-2 cortical microtubules exhibited aberrant and largely isotropic organization, reduced bundling and showed excessive branched microtubule formation. End-wise microtubule dynamics were not much affected, although a significantly slower rate of microtubule growth was measured in the ktn1-2 mutant where microtubule severing was completely abolished. KATANIN 1 depletion also brought about significant changes in preprophase microtubule band (PPB) organization and dynamics. In this case, many PPBs exhibited unisided organization and splayed appearance while in most cases they were broader than those of wild type cells. By recording PPB maturation, it was observed that PPBs in the mutant narrowed at a much slower pace compared to those in Col-0. The form of the mitotic spindle and the phragmoplast was not much affected in ktn1-2, however, the dynamics of both processes showed significant differences compared to wild type. In general, both mitosis and cytokinesis were considerably delayed in the mutant. Additionally, the mitotic spindle and the phragmoplast exhibited extensive rotational motions with the equatorial plane of the spindle being essentially uncoupled from the division plane set by the PPB. However, at the onset of its formation the phragmoplast undergoes rotational motion rectifying the expansion of the cell plate to match the original cell division plane. Conclusively, KATANIN 1 contributes to microtubule dynamics during interphase, regulates PPB formation and maturation and is involved in the positioning of the mitotic spindle and the phragmoplast.
Keywords: Arabidopsis; cell division; interphase; katanin; live imaging; microtubules; preprophase band; super resolution microscopy.
Figures





Similar articles
-
A Novel Katanin-Tethering Machinery Accelerates Cytokinesis.Curr Biol. 2019 Dec 2;29(23):4060-4070.e3. doi: 10.1016/j.cub.2019.09.049. Epub 2019 Nov 14. Curr Biol. 2019. PMID: 31735673
-
MOR1/MAP215 acts synergistically with katanin to control cell division and anisotropic cell elongation in Arabidopsis.Plant Cell. 2022 Jul 30;34(8):3006-3027. doi: 10.1093/plcell/koac147. Plant Cell. 2022. PMID: 35579372 Free PMC article.
-
A role for katanin in plant cell division: microtubule organization in dividing root cells of fra2 and lue1Arabidopsis thaliana mutants.Cytoskeleton (Hoboken). 2011 Jul;68(7):401-13. doi: 10.1002/cm.20522. Cytoskeleton (Hoboken). 2011. PMID: 21721142
-
Phosphorylation of Plant Microtubule-Associated Proteins During Cell Division.Front Plant Sci. 2019 Mar 11;10:238. doi: 10.3389/fpls.2019.00238. eCollection 2019. Front Plant Sci. 2019. PMID: 30915087 Free PMC article. Review.
-
Katanin: A Sword Cutting Microtubules for Cellular, Developmental, and Physiological Purposes.Front Plant Sci. 2017 Nov 21;8:1982. doi: 10.3389/fpls.2017.01982. eCollection 2017. Front Plant Sci. 2017. PMID: 29209346 Free PMC article. Review.
Cited by
-
Predicting Division Planes of Three-Dimensional Cells by Soap-Film Minimization.Plant Cell. 2018 Oct;30(10):2255-2266. doi: 10.1105/tpc.18.00401. Epub 2018 Aug 27. Plant Cell. 2018. PMID: 30150312 Free PMC article.
-
An anchoring complex recruits katanin for microtubule severing at the plant cortical nucleation sites.Nat Commun. 2021 Jun 17;12(1):3687. doi: 10.1038/s41467-021-24067-y. Nat Commun. 2021. PMID: 34140499 Free PMC article.
-
Multicolour three dimensional structured illumination microscopy of immunolabeled plant microtubules and associated proteins.Plant Methods. 2019 Mar 9;15:22. doi: 10.1186/s13007-019-0406-z. eCollection 2019. Plant Methods. 2019. PMID: 30899319 Free PMC article.
-
Finding a right place to cut: How katanin is targeted to cellular severing sites.Quant Plant Biol. 2022 Apr 11;3:e8. doi: 10.1017/qpb.2022.2. eCollection 2022. Quant Plant Biol. 2022. PMID: 37077970 Free PMC article. Review.
-
TANGLED1 mediates microtubule interactions that may promote division plane positioning in maize.J Cell Biol. 2020 Aug 3;219(8):e201907184. doi: 10.1083/jcb.201907184. J Cell Biol. 2020. PMID: 32568386 Free PMC article.
References
-
- Blancaflor E. B., Hasenstein K. H. (1993). Organization of cortical microtubules in graviresponding maize roots. Planta 191, 231–237. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

