Piper betle L. Modulates Senescence-Associated Genes Expression in Replicative Senescent Human Diploid Fibroblasts
- PMID: 28596968
- PMCID: PMC5449738
- DOI: 10.1155/2017/6894026
Piper betle L. Modulates Senescence-Associated Genes Expression in Replicative Senescent Human Diploid Fibroblasts
Abstract
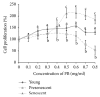
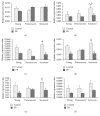
Piper betle (PB) is a traditional medicine that is widely used to treat different diseases around Asian region. The leaf extracts contain various bioactive compounds, which were reported to have antidiabetic, antibacterial, anti-inflammatory, antioxidant, and anticancer effects. In this study, the effect of PB aqueous extracts on replicative senescent human diploid fibroblasts (HDFs) was investigated by determining the expressions of senescence-associated genes using quantitative PCR. Our results showed that PB extracts at 0.4 mg/ml can improve cell proliferation of young (143%), presenescent (127.3%), and senescent (157.3%) HDFs. Increased expressions of PRDX6, TP53, CDKN2A, PAK2, and MAPK14 were observed in senescent HDFs compared to young and/or presenescent HDFs. Treatment with PB extracts modulates the transcriptional profile changes in senescent HDFs. By contrast, expressions of SOD1 increased, whereas GPX1, PRDX6, TP53, CDKN2A, PAK2, and MAPK14 were decreased in PB-treated senescent HDFs compared to untreated senescent HDFs. In conclusion, this study indicates the modulation of PB extracts on senescence-associated genes expression of replicative senescent HDFs. Further studies warrant determining the mechanism of PB in modulating replicative senescence of HDFs through these signaling pathways.
Figures


Similar articles
-
Targeting genes in insulin-associated signalling pathway, DNA damage, cell proliferation and cell differentiation pathways by tocotrienol-rich fraction in preventing cellular senescence of human diploid fibroblasts.Clin Ter. 2015 Nov-Dec;166(6):e365-73. doi: 10.7417/T.2015.1902. Clin Ter. 2015. PMID: 26794818
-
Chlorella vulgaris modulates the expression of senescence-associated genes in replicative senescence of human diploid fibroblasts.Mol Biol Rep. 2020 Jan;47(1):369-379. doi: 10.1007/s11033-019-05140-8. Epub 2019 Oct 22. Mol Biol Rep. 2020. PMID: 31642042
-
Comparative effect of Piper betle, Chlorella vulgaris and tocotrienol-rich fraction on antioxidant enzymes activity in cellular ageing of human diploid fibroblasts.BMC Complement Altern Med. 2013 Aug 16;13:210. doi: 10.1186/1472-6882-13-210. BMC Complement Altern Med. 2013. PMID: 23948056 Free PMC article.
-
The underlying mechanism of action for various medicinal properties of Piper betle (betel).Clin Ter. 2015;166(5):208-14. doi: 10.7417/CT.2015.1880. Clin Ter. 2015. PMID: 26550811 Review.
-
Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints.Ann N Y Acad Sci. 2000 Jun;908:111-25. doi: 10.1111/j.1749-6632.2000.tb06640.x. Ann N Y Acad Sci. 2000. PMID: 10911952 Review.
Cited by
-
Modulation of Cellular Senescence in HEK293 and HepG2 Cells by Ultrafiltrates UPla and ULu Is Partly Mediated by Modulation of Mitochondrial Homeostasis under Oxidative Stress.Int J Mol Sci. 2023 Apr 4;24(7):6748. doi: 10.3390/ijms24076748. Int J Mol Sci. 2023. PMID: 37047720 Free PMC article.
-
Effect of Antioxidants on the Fibroblast Replicative Lifespan In Vitro.Oxid Med Cell Longev. 2020 Sep 23;2020:6423783. doi: 10.1155/2020/6423783. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33029282 Free PMC article. Review.
-
Influence of Piper betle L. extract on umbilical cord cells in vitro and potential treating cutaneous wound.Heliyon. 2021 Mar 8;7(3):e06248. doi: 10.1016/j.heliyon.2021.e06248. eCollection 2021 Mar. Heliyon. 2021. PMID: 33748448 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

