Soluble Urokinase Receptor and the Kidney Response in Diabetes Mellitus
- PMID: 28596971
- PMCID: PMC5449757
- DOI: 10.1155/2017/3232848
Soluble Urokinase Receptor and the Kidney Response in Diabetes Mellitus
Abstract
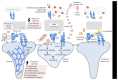
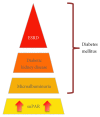
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease (ESRD) worldwide. DN typically manifests by glomerular hyperfiltration and microalbuminuria; then, the disease progresses to impaired glomerular filtration rate, which leads to ESRD. Treatment options for DN include the strict control of blood glucose levels and pressure (e.g., intraglomerular hypertension). However, the search for novel therapeutic strategies is ongoing. These include seeking specific molecules that contribute to the development and progression of DN to potentially interfere with these "molecular targets" as well as with the cellular targets within the kidney such as podocytes, which play a major role in the pathogenesis of DN. Recently, podocyte membrane protein urokinase receptor (uPAR) and its circulating form (suPAR) are found to be significantly induced in glomeruli and sera of DN patients, respectively, and elevated suPAR levels predicted diabetic kidney disease years before the occurrence of microalbuminuria. The intent of this review is to summarize the emerging evidence of uPAR and suPAR in the clinical manifestations of DN. The identification of specific pathways that govern DN will help us build a more comprehensive molecular model for the pathogenesis of the disease that can inform new opportunities for treatment.
Figures
References
-
- Eknoyan G., Hostetter T., Bakris G. L., et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK) American Journal of Kidney Diseases. 2003;42(4):617–622. doi: 10.1016/S0272-6386(03)00826-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

