Biodegradable Polyphosphazene-Based Blends for Regenerative Engineering
- PMID: 28596987
- PMCID: PMC5459410
- DOI: 10.1007/s40883-016-0022-7
Biodegradable Polyphosphazene-Based Blends for Regenerative Engineering
Abstract
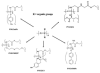
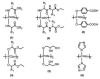
The occurrence of musculoskeletal tissue injury or disease and the subsequent functional impairment is at an alarming rate. It continues to be one of the most challenging problems in the human health care. Regenerative engineering offers a promising transdisciplinary strategy for tissues regeneration based on the convergence of tissue engineering, advanced materials science, stem cell science, developmental biology and clinical translation. Biomaterials are emerging as extracellular-mimicking matrices designed to provide instructive cues to control cell behavior and ultimately, be applied as therapies to regenerate damaged tissues. Biodegradable polymers constitute an attractive class of biomaterials for the development of scaffolds due to their flexibility in chemistry and the ability to be excreted or resorbed by the body. Herein, the focus will be on biodegradable polyphosphazene-based blend systems. The synthetic flexibility of polyphosphazene, combined with the unique inorganic backbone, has provided a springboard for more research and subsequent development of numerous novel materials that are capable of forming miscible blends with poly (lactide-co-glycolide) (PLAGA). Laurencin and co-workers has demonstrated the exploitation of the synthetic flexibility of Polyphosphazene that will allow the design of novel polymers, which can form miscible blends with PLAGA for biomedical applications. These novel blends, due to their well-tuned biodegradability, and mechanical and biological properties coupled with the buffering capacity of the degradation products, constitute ideal materials for regeneration of various musculoskeletal tissues.
Lay summary: Regenerative engineering aims to regenerate complex tissues to address the clinical challenge of organ damage. Tissue engineering has largely focused on the restoration and repair of individual tissues and organs, but over the past 25 years, scientific, engineering, and medical advances have led to the introduction of this new approach which involves the regeneration of complex tissues and biological systems such as a knee or a whole limb. While a number of excellent advanced biomaterials have been developed, the choice of biomaterials, however, has increased over the past years to include polymers that can be designed with a range of mechanical properties, degradation rates, and chemical functionality. The polyphosphazenes are one good example. Their chemical versatility and hydrogen bonding capability encourages blending with other biologically relevant polymers. The further development of Polyphosphazene-based blends will present a wide spectrum of advanced biomaterials that can be used as scaffolds for regenerative engineering and as well as other biomedical applications.
Keywords: Biodegradable polymers; Dipeptide-based Polyphosphazene; Musculoskeletal; Polyphosphazene Blends; Regenerative engineering.
Figures



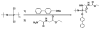
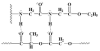




References
-
- Laurencin CT, Khan Y. Regenerative engineering. Science translational medicine. 2012;4(160):160ed169–160ed169. - PubMed
-
- Polyphosphazenes for Biomedical Applications (1) Hoboken, US: Wiley; 2009.
-
- Allcock HR, Morozowich NL. Bioerodible polyphosphazenes and their medical potential. Polymer Chemistry. 2012;3(3):578–590.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
