Oxidative Phosphorylation as a Target Space for Tuberculosis: Success, Caution, and Future Directions
- PMID: 28597820
- PMCID: PMC5480969
- DOI: 10.1128/microbiolspec.TBTB2-0014-2016
Oxidative Phosphorylation as a Target Space for Tuberculosis: Success, Caution, and Future Directions
Abstract
The emergence and spread of drug-resistant pathogens, and our inability to develop new antimicrobials to combat resistance, have inspired scientists to seek out new targets for drug development. The Mycobacterium tuberculosis complex is a group of obligately aerobic bacteria that have specialized for inhabiting a wide range of intracellular and extracellular environments. Two fundamental features in this adaptation are the flexible utilization of energy sources and continued metabolism in the absence of growth. M. tuberculosis is an obligately aerobic heterotroph that depends on oxidative phosphorylation for growth and survival. However, several studies are redefining the metabolic breadth of the genus. Alternative electron donors and acceptors may provide the maintenance energy for the pathogen to maintain viability in hypoxic, nonreplicating states relevant to latent infection. This hidden metabolic flexibility may ultimately decrease the efficacy of drugs targeted against primary dehydrogenases and terminal oxidases. However, it may also open up opportunities to develop novel antimycobacterials targeting persister cells. In this review, we discuss the progress in understanding the role of energetic targets in mycobacterial physiology and pathogenesis and the opportunities for drug discovery.
Figures
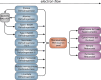
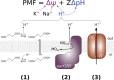
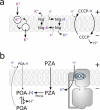
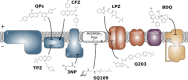
References
-
- Brodie AF, Gutnik DL (ed). 1972. Electron Transport and Oxidative Phosphorylation in Microbial Systems. Marcel Dekker Inc., New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

