Celecoxib for rheumatoid arthritis
- PMID: 28597983
- PMCID: PMC6481589
- DOI: 10.1002/14651858.CD012095.pub2
Celecoxib for rheumatoid arthritis
Abstract
Background: Rheumatoid arthritis is a systemic auto-immune disorder that causes widespread and persistent inflammation of the synovial lining of joints and tendon sheaths. Presently, there is no cure for rheumatoid arthritis and treatment focuses on managing symptoms such as pain, stiffness and mobility, with the aim of achieving stable remission and improving mobility. Celecoxib is a selective non-steroidal anti-inflammatory drug (NSAID) used for treatment of people with rheumatoid arthritis.
Objectives: To assess the benefits and harms of celecoxib in people with rheumatoid arthritis.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and clinical trials registers (ClinicalTrials.gov and the World Health Organization trials portal) to May 18, 2017. We also searched the reference and citation lists of included studies.
Selection criteria: We included prospective randomized controlled trials (RCTs) that compared oral celecoxib (200 mg and 400 mg daily) versus no intervention, placebo or a traditional NSAID (tNSAID) in people with confirmed rheumatoid arthritis, of any age and either sex. We excluded studies with fewer than 50 participants in each arm or had durations of fewer than four weeks treatment.
Data collection and analysis: We used standard methodological procedures expected by The Cochrane Collaboration.
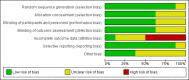
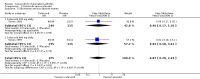
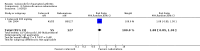
Main results: We included eight RCTs with durations of 4 to 24 weeks, published between 1998 and 2014 that involved a total of 3988 adults (mean age = 54 years), most of whom were women (73%). Participants had rheumatoid arthritis for an average of 9.2 years. All studies were assessed at high or unclear risk of bias in at least one domain. Overall, evidence was assessed as moderate-to-low quality. Five studies were funded by pharmaceutical companies. Celecoxib versus placeboWe included two studies (N = 873) in which participants received 200 mg daily or 400 mg daily or placebo. Participants who received celecoxib showed significant clinical improvement compared with those receiving placebo (15% absolute improvement; 95% CI 7% to 25%; RR 1.53, 95% CI 1.25 to 1.86; number needed to treat to benefit (NNTB) = 7, 95% CI 5 to 13; 2 studies, 873 participants; moderate to low quality evidence).Participants who received celecoxib reported less pain than placebo-treated people (11% absolute improvement; 95% CI 8% to 14%; NNTB = 4, 95% CI 3 to 6; 1 study, 706 participants) but results were inconclusive for improvement in physical function (MD -0.10, 95% CI 0.29 to 0.10; 1 study, 706 participants).In the celecoxib group, 15/293 participants developed ulcers, compared with 4/99 in the placebo group (Peto OR 1.26, 95% CI 0.44 to 3.63; 1 study, 392 participants; low quality evidence). Nine (of 475) participants in the celecoxib group developed short-term serious adverse events, compared with five (of 231) in the placebo group (Peto OR 0.87 (0.28 to 2.69; 1 study, 706 participants; low quality evidence).There were fewer withdrawals among people who received celecoxib (163/475) compared with placebo (130/231) (22% absolute change; 95% CI 16% to 27%; RR 0.61, 95% CI 0.52 to 0.72; 1 study, 706 participants).Cardiovascular events (myocardial infarction, stroke) were not reported. However, regulatory agencies warn of increased cardiovascular event risk associated with celecoxib. Celecoxib versus tNSAIDsSeven studies (N = 2930) compared celecoxib and tNSAIDs (amtolmetin guacyl, diclofenac, ibuprofen, meloxicam, nabumetone, naproxen, pelubiprofen); one study included comparisons of both placebo and tNSAIDs (N = 1149).There was a small improvement, which may not be clinically significant, in numbers of participants achieving ACR20 criteria response in the celecoxib group compared to tNSAIDs (4% absolute improvement; 95% CI 0% less improvement to 8% more improvement; RR 1.10, 95% CI 0.99 to 1.23; 4 studies, 1981 participants). There was a lack of evidence of difference between participants in the celecoxib and tNSAID groups in terms of pain or physical function. Results were assessed at moderate-to-low quality evidence (downgraded due to risk of bias and inconsistency).People who received celecoxib had a lower incidence of gastroduodenal ulcers ≥ 3 mm (34/870) compared with those who received tNSAIDs (116/698). This corresponded to 12% absolute change (95% CI 11% to 13%; RR 0.22, 95% CI 0.15 to 0.32; 5 studies, 1568 participants; moderate quality evidence). There were 7% fewer withdrawals among people who received celecoxib (95% CI 4% to 9%; RR 0.73, 95% CI 0.62 to 0.86; 6 studies, 2639 participants).Results were inconclusive for short-term serious adverse events and cardiovascular events (low quality evidence). There were 17/918 serious adverse events in people taking celecoxib compared to 42/1236 among people who received placebo (Peto OR 0.71; 95% CI 0.39 to 1.28; 5 studies, 2154 participants). Cardiovascular events were reported in both celecoxib and placebo groups in one study (149 participants).
Authors' conclusions: Celecoxib may improve clinical symptoms, alleviate pain and contribute to little or no difference in physical function compared with placebo. Celecoxib was associated with fewer numbers of participant withdrawals. Results for incidence of gastroduodenal ulcers (≥ 3 mm) and short-term serious adverse events were uncertain; however, there were few reported events for either.Celecoxib may slightly improve clinical symptoms compared with tNSAIDs. Results for reduced pain and improved physical function were uncertain. Particpants taking celecoxib had lower incidence of gastroduodenal ulcers (≥ 3 mm) and there were fewer withdrawals from trials. Results for cardiovascular events and short-term serious adverse events were also uncertain.Uncertainty about the rate of cardiovascular events between celecoxib and tNSAIDs could be due to risk of bias; another factor is that these were small, short-term trials. It has been reported previously that both celecoxib and tNSAIDs increase cardiovascular event rates. Our confidence in results about harms is therefore low. Larger head-to-head clinical trials comparing celecoxib to other tNSAIDs is needed to better inform clinical practice.
Conflict of interest statement
Mahir Fidahic: no conflict of interest. Antonia Jelicic Kadic: no conflict of interest. Mislav Radic: no conflict of interest. Livia Puljak: no conflict of interest.
Figures
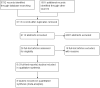



























































Update of
References
References to studies included in this review
Choi 2014 {published data only}
-
- Choi IA, Baek CS, Lee YA, Chung WT, Park YE, Lee YJ, et al. Comparison of the efficacy and safety profiles of a pelubiprofen versus celecoxib in patients with rheumatoid arthritis: a 6‐week, multicenter, randomised, double‐blind, phase III, non‐inferiority clinical trial. BMC Musculosceletal Disorders 2014;15(357):1‐9. - PMC - PubMed
Emery 1999 {published data only}
-
- Emery P, Zeider H, Kvien TK, Guslandi M, Naudin R, Stead H, et al. Celecoxib versus diclofenac in long‐term management of rheumatoid arthritis: randomised double‐blind comparison. Lancet 1999;354(9196):2106‐11. - PubMed
Goldstein 2001 {published data only}
-
- Goldstein JL, Correa P, Zhao WW, Burr AM, Hubbard RC, Verburg KM, et al. Reduced incidence of gastroduodenal ulcers with celecoxib, a novel cyclooxygenase‐2 inhibitor, compared to naproxen in patients with arthritis. American Journal of Gastroenterology 2001;96(4):1019‐27. - PubMed
Jajic 2005 {published data only}
-
- Jajić Z, Malaise M, Nekam K, Koó E, Dankó K, Kovacs M, et al. Gastrointestinal safety of amtolmetin guacyl in comparison with celecoxib in patients with rheumatoid arthritis. Clinical and Experimental Rheumatology 2005;23(6):809‐818. - PubMed
Kivitz 2004 {published data only}
-
- Kivitz AJ, Nayiager S, Schimansky T, Gimona A, Thurston HJ, Hawkey C. Reduced incidence of gastroduodenal ulcers associated with lumiracoxib compared with ibuprofen in patients with rheumatoid arthritis. Alimentary Pharmacology and Therapeutics 2004;19(11):1189‐98. - PubMed
Shi 2004 {published data only}
-
- Shi W, Wang YM, Li LS, Yan M, Li D, Chen NN, et al. Safety and efficacy of oral nonsteroidal anti‐inflammatory drugs in patients with rheumatoid arthritis. Clinical Drug Investigation 2004;2(24):89‐101. - PubMed
Simon 1998 {published data only}
-
- Simon LS, Lanza FL, Lipsky PE, Hubbard RC, Talwalker S, Schwartz BD, et al. Preliminary study of the safety and efficacy of SC‐58635, a novel cyclooxygenase 2 inhibitor: efficacy and safety in two placebo‐controlled trials in osteoarthritis and rheumatoid arthritis, and studies of gastrointestinal and platelet effects. Arthritis and Rheumatism 1998;41(9):1591‐602. - PubMed
Simon 1999 {published data only}
-
- Simon LS, Weaver AL, Graham DY, Kivitz AJ, Lipsky PE, Hubbart RC, et al. Anti‐inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis. JAMA 1999;282(20):1921‐8. - PubMed
-
- Zhao SZ, Fiechtner JI, Tindall EA, Dedhiya SD, Zhao WW, Osterhaus JT, et al. Evaluation of health‐related quality of life of rheumatoid arthritis patients treated with celecoxib. Arthritis Care and Research 2000;12(2):112‐21. - PubMed
References to studies excluded from this review
Chan 2002 {published data only}
-
- Chan FKL, Hung LCT, Suen BY, Wu JCY, Lee KC, Leung VKS, et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. New England Journal of Medicine 2002;347(26):2104‐10. - PubMed
Chan 2004 {published data only}
-
- Chan FKL, Hung LCT, Suen BY, Wong VWS, Hui AJ, Wu JCY, et al. Celecoxib versus diclofenac plus omeprazole in high‐risk arthritis patients: results of a randomized double‐blind trial. Gastroenterology 2004;127(4):1038‐43. - PubMed
Chan 2007 {published data only}
-
- Chan FKL, Wong VWS, Suen BY, Wu JCY, Ching JYL, Hung LCT, et al. Combination of a cyclo‐oxygenase‐2 inhibitor and a proton‐pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double‐blind, randomised trial. Lancet 2007;369(9573):1621‐6. - PubMed
Chan 2010 {published data only}
-
- Chan FKL, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR):a randomised trial. Lancet 2010;376(9736):173‐9. - PubMed
Chan 2015 {published data only}
-
- Chan FKL, Ching J, Cheung C, Lam LYK, Au KWL, Ng SC, et al. Prevention of recurrent ulcer bleeding in arthritis patients with high cardiovascular and high gastrointestinal risks: an 18‐month, double‐blind, randomized trial. Gastroenterology 2015;148:157‐158.
Cheatum 1999 {published data only}
-
- Cheatum DE, Arvanitakis C, Gumpel M, Stead H, Geis GS. An endoscopic study of gastroduodenal lesions induced by nonsteroidal anti‐inflammatory drugs. Clinical Therapeutics 1999;21(6):992‐1003. - PubMed
Cheung 2010 {published data only}
-
- Cheung R, Cheng TT, Dong Y, Lin HY, Lai K, Lau C, et al. Incidence of gastroduodenal ulcers during treatment with celecoxib or diclofenac: pooled results from three 12‐week trials in Chinese patients with osteoarthritis or rheumatoid arthritis. International Journal of Rheumatic Diseases 2010;13(2):151‐7. - PubMed
Goldstein 2002 {published data only}
-
- Goldstein JL, Eisen GM, Burke TA, Pena BM, Lefkowith J, Geis GS. Dyspepsia tolerability from the patients’ perspective: a comparison of celecoxib with diclofenac. Alimentary Pharmacology and Therapeutics 2002;16(4):819‐27. - PubMed
Hegazy 2011 {published data only}
Kellner 2012 {published data only}
-
- Kellner HL, Li C, Essex MN. Efficacy and safety of celecoxib versus diclofenac and omeprazole in elderly arthritis patients: a subgroup analysis of the CONDOR trial. Current Medical Research and Opinion 2012;28(9):1537‐45. - PubMed
Kellner 2013 {published data only}
Laine 2002 {published data only}
-
- Laine L, Bombardier C, Hawkey CJ, Davis B, Shapiro D, Brett C, et al. Stratifying the risk of NSAID‐related upper gastrointestinal clinical events: results of a double‐blind outcomes study in patients with rheumatoid arthritis. Gastroenterology 2002;123(4):1006‐12. - PubMed
Laine 2007 {published data only}
-
- Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long‐term (MEDAL) programme: a randomised comparison. Lancet 2007;369(9560):465‐73. - PubMed
Liu 2015 {published data only}
-
- Liu D, Guo M, Hu Y, Liu T, Yan J, Luo Y, et al. Effect of Sanhuangwuji powder, anti‐rheumatic drugs, and ginger‐partitioned acupoint stimulation on the treatment of rheumatoid arthritis with peptic ulcer: a randomized controlled study. Journal of Traditional Chinese Medicine 2015;35(3):273‐80. - PubMed
Nissen 2016 {unpublished data only}
-
- Nissen SE, Yeomans ND, Solomon DH, Lüscher TF, Libby P, Husni ME, et al. PRECISION Trial Investigators. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. New England Journal of Medicine 2016;375(26):2519‐29. - PubMed
Silverstein 2000 {published data only}
-
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis the CLASS study: a randomized controlled trial. JAMA 2000;284(10):1247‐55. - PubMed
Song 2007 {published data only}
-
- Song YW, Lee EY, Koh EM, Cha HS, Yoo B, Lee CK, et al. Assessment of comparative pain relief and tolerability of SK1306X compared with celecoxib in patients with rheumatoid arthritis: a 6‐week, multicenter, randomized, double‐blind, double‐dummy, phase lll, noninferiority clinical trial. Clinical Therapeutics 2007;29(5):862‐73. - PubMed
Zayat 2011 {published data only}
-
- Zayat AS, Conaghan PG, Sharif M, Freeston JE, Wenham C, Hensor EMA, et al. Do non‐steroidal anti‐inflammatory drugs have a significant effect on detection and grading of ultrasound‐detected synovitis in patients with rheumatoid arthritis? Results from a randomised study. Annals of the Rheumatic Diseases 2011;70(10):1746‐51. - PubMed
Additional references
Akl 2011
Aletaha 2005
-
- Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis and Rheumatism 2005;52(9):2625‐36. - PubMed
Aletaha 2010
-
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and Rheumatism 2010;62(9):2569‐81. - PubMed
Arnett 1988
-
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31(3):315‐24. - PubMed
Ashroft 2001
-
- Ashcroft DM, Chapman SR, Clark WK, Millson DS. Upper gastroduodenal ulceration in arthritis patients treated with celecoxib. Annals of Pharmacotherapy 2001;35(7‐8):829‐34. - PubMed
Bensen 2000
-
- Bensen WG, Zhao SZ, Burke TA, Zabinski RA, Makuch RW, Maurath CJ, et al. Uper gastrointestinal tolerability of celecoxib, a COX‐2 specific inhibitor, compared to naproxen and placebo. Journal of Rheumatology 2000;27(8):1876‐83. - PubMed
Cates 2008
-
- Cates C. Dr Chris Cates’ EBM website. Visual Rx Version 3. www.nntonline.net/visualrx/ (accessed prior to 10 April 2017).
Chou 2006
-
- Chou R, Helfand M, Peterson K, Dana T, Roberts C. Drug class review on cyclo‐oxygenase (COX)‐2 inhibitors and non‐steroidal anti‐inflammatory drugs (NSAIDs): Final Report Update 3 [Internet]. www.ncbi.nlm.nih.gov/books/NBK10620/ (accessed prior to 10 April 2017).
CNT 2013
Conaghan 2012
Cramp 2013
Cross 2014
-
- Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Annals of the Rheumatic Diseases 2014;73(7):1316‐22. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
FDA 2016
-
- USA Food, Drug Administration (FDA). Celebrex product information. www.accessdata.fda.gov/drugsatfda_docs/label/2005/020998s017lbl.pdf (accessed 11 December 2016).
Felson 1995
-
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1995;38(6):727‐35. - PubMed
Felson 1998
-
- Felson DT, Anderson JJ, Lange ML, Wells G, LaValley MP. Should improvement in rheumatoid arthritis clinical trials be defined as fifty percent or seventy percent improvement in core set measures, rather than twenty percent?. Arthritis and Rheumatism 1998;41(9):1564‐70. - PubMed
Gardiner 1993
-
- Gardiner PV, Sykes HR, Hassey GA, Walker DJ. An evaluation of the health assessment questionnaire in long‐term longitudinal follow‐up of disability in rheumatoid arthritis. British Journal of Rheumatology 1993;32(8):724‐8. - PubMed
Ghogomu 2014
-
- Ghogomu EA, Maxwell LJ, Buchbinder R, Rader T, Pardo Pardo J, Johnston RV, et al. Updated method guidelines for Cochrane musculoskeletal group systematic reviews and meta analyses. Journal of Rheumatology 2014;41(2):194‐205. - PubMed
GRADEpro GDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hawkey 1999
-
- Hawkey CJ. Cox‐2 inhibitors. Lancet 1999;353(9149):307‐14. - PubMed
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hurkmans 2009
Kearney 2006
Lanas 2014
-
- Lanas Á, Carrera‐Lasfuentes P, Arguedas Y, García S, Bujanda L, Calvet X, et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti‐inflammatory drugs, antiplatelet agents, or anticoagulants. Clinical Gastroenterology and Hepatology 2014;13(5):906‐12. - PubMed
Lethaby 2013
Lopez‐Olivo 2014
Lopez‐Olivo 2015
McInnes 2011
-
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. New England Journal of Medicine 2011;365(23):2205‐19. - PubMed
Moore 2013
Myasoedova 2010
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 2012a
Richards 2012b
Roubille 2015
-
- Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors,methotrexate, non‐steroidal anti‐inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis:a systematic review and meta‐analysis. Annals of the Rheumatic Diseases 2015;74(3):480‐9. - PMC - PubMed
Ruiz Garcia 2014
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann H, Oxman AD, Vist GE, Higgins JBT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Scott 2010
-
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376(9746):1094‐108. - PubMed
Singer 2010
-
- Singer JB, Lewitzky S, Leroy E, Yang F, Zhao X, Klickstein L, et al. A genome‐wide study identifies HLA alleles associated with lumiracoxib‐related liver injury. Nature Genetics 2010;42(8):711‐4. - PubMed
Süleyman 2007
-
- Süleyman H, Demircan B, Karagöz Y. Anti‐inflammatory and side effects of cyclooxygenase inhibitors. Pharmacological Reports 2007;59(3):247‐58. - PubMed
TGA 2010
-
- Therapeutic Goods Administration (Australia). Australian Public Assessment Report for Celecoxib. Proprietary Product Name: Celebrex; Sponsor: Pfizer Australia Pty Ltd; August 2010. www.tga.gov.au/sites/default/files/auspar‐celebrex.pdf (accessed 11 December 2016). [Web resource]
Trelle 2011
Van Walsem 2015
-
- Walsem A, Pandhi S, Nixon RM, Guyot P, Karabis A. Relative benefit‐risk comparing diclofenac to other traditional non‐steroidal anti‐inflammatory drugs and cyclooxygenase‐2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta‐analysis. Arthritis Research and Therapy 2015;17(66):1‐18. - PMC - PubMed
Verhagen 2015
Vucic 2015
-
- Vucic K, Jelicic Kadic A, Puljak L. Survey of Cochrane protocols found methods for data extraction from figures not mentioned or unclear. Journal of Clinical Epidemiology 2015; Vol. 66, issue 10:1161‐4. - PubMed
References to other published versions of this review
Fidahic 2016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

