Carbon Ion Radiotherapy: A Review of Clinical Experiences and Preclinical Research, with an Emphasis on DNA Damage/Repair
- PMID: 28598362
- PMCID: PMC5483885
- DOI: 10.3390/cancers9060066
Carbon Ion Radiotherapy: A Review of Clinical Experiences and Preclinical Research, with an Emphasis on DNA Damage/Repair
Abstract
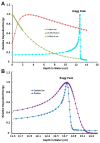
Compared to conventional photon-based external beam radiation (PhXRT), carbon ion radiotherapy (CIRT) has superior dose distribution, higher linear energy transfer (LET), and a higher relative biological effectiveness (RBE). This enhanced RBE is driven by a unique DNA damage signature characterized by clustered lesions that overwhelm the DNA repair capacity of malignant cells. These physical and radiobiological characteristics imbue heavy ions with potent tumoricidal capacity, while having the potential for simultaneously maximally sparing normal tissues. Thus, CIRT could potentially be used to treat some of the most difficult to treat tumors, including those that are hypoxic, radio-resistant, or deep-seated. Clinical data, mostly from Japan and Germany, are promising, with favorable oncologic outcomes and acceptable toxicity. In this manuscript, we review the physical and biological rationales for CIRT, with an emphasis on DNA damage and repair, as well as providing a comprehensive overview of the translational and clinical data using CIRT.
Keywords: DNA repair; carbon therapy; complex DNA damage; hadron therapy; proton therapy; radiation oncology.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

