Plant Lectins Targeting O-Glycans at the Cell Surface as Tools for Cancer Diagnosis, Prognosis and Therapy
- PMID: 28598369
- PMCID: PMC5486055
- DOI: 10.3390/ijms18061232
Plant Lectins Targeting O-Glycans at the Cell Surface as Tools for Cancer Diagnosis, Prognosis and Therapy
Abstract
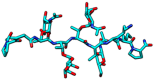
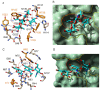
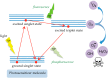
Aberrant O-glycans expressed at the surface of cancer cells consist of membrane-tethered glycoproteins (T and Tn antigens) and glycolipids (Lewis a, Lewis x and Forssman antigens). All of these O-glycans have been identified as glyco-markers of interest for the diagnosis and the prognosis of cancer diseases. These epitopes are specifically detected using T/Tn-specific lectins isolated from various plants such as jacalin from Artocarpus integrifola, and fungi such as the Agaricus bisporus lectin. These lectins accommodate T/Tn antigens at the monosaccharide-binding site; residues located in the surrounding extended binding-site of the lectins often participate in the binding of more extended epitopes. Depending on the shape and size of the extended carbohydrate-binding site, their fine sugar-binding specificity towards complex O-glycans readily differs from one lectin to another, resulting in a great diversity in their sugar-recognition capacity. T/Tn-specific lectins have been extensively used for the histochemical detection of cancer cells in biopsies and for the follow up of the cancer progression and evolution. T/Tn-specific lectins also induce a caspase-dependent apoptosis in cancer cells, often associated with a more or less severe inhibition of proliferation. Moreover, they provide another potential source of molecules adapted to the building of photosensitizer-conjugates allowing a specific targeting to cancer cells, for the photodynamic treatment of tumors.
Keywords: Morniga G; O-glycosylation; T antigen; Tn antigen; cancer; diagnosis; lectin; peanut lectin; photodynamic therapy; prognosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Recognition profile of Morus nigra agglutinin (Morniga G) expressed by monomeric ligands, simple clusters and mammalian polyvalent glycotopes.Mol Immunol. 2007 Jan;44(4):451-62. doi: 10.1016/j.molimm.2006.02.017. Epub 2006 Apr 3. Mol Immunol. 2007. PMID: 16581130
-
Morniga-G, a T/Tn-Specific Lectin, Induces Leukemic Cell Death via Caspase and DR5 Receptor-Dependent Pathways.Int J Mol Sci. 2019 Jan 8;20(1):230. doi: 10.3390/ijms20010230. Int J Mol Sci. 2019. PMID: 30626136 Free PMC article.
-
Carbohydrate recognition factors of a Talpha (Galbeta1-->3GalNAcalpha1-->Ser/Thr) and Tn (GalNAcalpha1-->Ser/Thr) specific lectin isolated from the seeds of Artocarpus lakoocha.Glycobiology. 2005 Jan;15(1):67-78. doi: 10.1093/glycob/cwh144. Epub 2004 Aug 25. Glycobiology. 2005. PMID: 15329360
-
Legume Lectins with Different Specificities as Potential Glycan Probes for Pathogenic Enveloped Viruses.Cells. 2022 Jan 20;11(3):339. doi: 10.3390/cells11030339. Cells. 2022. PMID: 35159151 Free PMC article. Review.
-
The Cosmc connection to the Tn antigen in cancer.Cancer Biomark. 2014 Jan 1;14(1):63-81. doi: 10.3233/CBM-130375. Cancer Biomark. 2014. PMID: 24643043 Free PMC article. Review.
Cited by
-
Jacalin-Curcumin Complex Sensitizes the Breast Cancer MDA-MB-231 Cell Line.Int J Mol Sci. 2023 Dec 12;24(24):17399. doi: 10.3390/ijms242417399. Int J Mol Sci. 2023. PMID: 38139227 Free PMC article.
-
Sialyl-Tn Antigen-Imprinted Dual Fluorescent Core-Shell Nanoparticles for Ratiometric Sialyl-Tn Antigen Detection and Dual-Color Labeling of Cancer Cells.ACS Appl Nano Mater. 2022 Dec 23;5(12):17592-17605. doi: 10.1021/acsanm.2c03252. Epub 2022 Nov 16. ACS Appl Nano Mater. 2022. PMID: 36583127 Free PMC article.
-
Characterization of a Novel Mannose-Binding Lectin with Antiviral Activities from Red Alga, Grateloupia chiangii.Biomolecules. 2020 Feb 19;10(2):333. doi: 10.3390/biom10020333. Biomolecules. 2020. PMID: 32092955 Free PMC article.
-
Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer.Int J Mol Sci. 2023 Jun 14;24(12):10120. doi: 10.3390/ijms241210120. Int J Mol Sci. 2023. PMID: 37373268 Free PMC article. Review.
-
Chemoenzymatic Tagging of Tn/TF/STF Antigens in Living Systems.Isr J Chem. 2023 Oct;63(10-11):e202300081. doi: 10.1002/ijch.202300081. Epub 2023 Sep 15. Isr J Chem. 2023. PMID: 38737670 Free PMC article.
References
-
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989;52:257–331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

