The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism
- PMID: 28598392
- PMCID: PMC5486060
- DOI: 10.3390/ijms18061237
The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism
Abstract
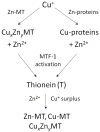
Recent discoveries in zinc biology provide a new platform for discussing the primary physiological functions of mammalian metallothioneins (MTs) and their exquisite zinc-dependent regulation. It is now understood that the control of cellular zinc homeostasis includes buffering of Zn2+ ions at picomolar concentrations, extensive subcellular re-distribution of Zn2+, the loading of exocytotic vesicles with zinc species, and the control of Zn2+ ion signalling. In parallel, characteristic features of human MTs became known: their graded affinities for Zn2+ and the redox activity of their thiolate coordination environments. Unlike the single species that structural models of mammalian MTs describe with a set of seven divalent or eight to twelve monovalent metal ions, MTs are metamorphic. In vivo, they exist as many species differing in redox state and load with different metal ions. The functions of mammalian MTs should no longer be considered elusive or enigmatic because it is now evident that the reactivity and coordination dynamics of MTs with Zn2+ and Cu⁺ match the biological requirements for controlling-binding and delivering-these cellular metal ions, thus completing a 60-year search for their functions. MT represents a unique biological principle for buffering the most competitive essential metal ions Zn2+ and Cu⁺. How this knowledge translates to the function of other families of MTs awaits further insights into the specifics of how their properties relate to zinc and copper metabolism in other organisms.
Keywords: affinity; copper; metallothionein; metamorphic proteins; thionein; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Margoshes M., Vallee B.L. A cadmium protein from equine kidney cortex. J. Am. Chem. Soc. 1957;79:4813–4814. doi: 10.1021/ja01574a064. - DOI
-
- Kägi J.H., Vallee B.L. Metallothionein: A cadmium- and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1960;235:3460–3465. - PubMed
-
- Li Y., Maret W. Human metallothionein metallomics. J. Anal. At. Spectrom. 2008;23:1055–1062. doi: 10.1039/b802220h. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

