DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity
- PMID: 28598415
- PMCID: PMC5472757
- DOI: 10.1038/ncomms15618
DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity
Abstract
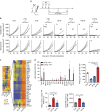
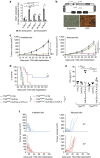
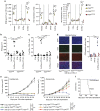
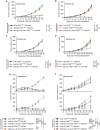
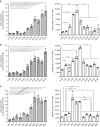
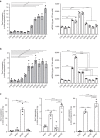
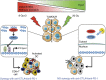
Radiotherapy is under investigation for its ability to enhance responses to immunotherapy. However, the mechanisms by which radiation induces anti-tumour T cells remain unclear. We show that the DNA exonuclease Trex1 is induced by radiation doses above 12-18 Gy in different cancer cells, and attenuates their immunogenicity by degrading DNA that accumulates in the cytosol upon radiation. Cytosolic DNA stimulates secretion of interferon-β by cancer cells following activation of the DNA sensor cGAS and its downstream effector STING. Repeated irradiation at doses that do not induce Trex1 amplifies interferon-β production, resulting in recruitment and activation of Batf3-dependent dendritic cells. This effect is essential for priming of CD8+ T cells that mediate systemic tumour rejection (abscopal effect) in the context of immune checkpoint blockade. Thus, Trex1 is an upstream regulator of radiation-driven anti-tumour immunity. Trex1 induction may guide the selection of radiation dose and fractionation in patients treated with immunotherapy.
Conflict of interest statement
The authors declare no competing financial interests related to this manuscript, but S.C.F. has received speaker compensation from Bristol-Myer Squibb, Sanofi, Regeneron, Varian, Elekta and Janssen, and S.D. currently serves as a consultant for Eisai, Inc, Lytix Biopharma and Nanobiotix.
Figures


References
-
- Sharma P. & Allison J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015). - PubMed
-
- Demaria S. et al.. Immune-mediated inhibition of metastases following treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

